By: Kyle Richardville, Understanding Ag, LLC
About the “Understanding” series
Agriculture isn’t rocket science. It’s much more complex than that. Farming and ranching involve the fields of biology, ecology, chemistry, botany, physics, geology, meteorology, politics, economics, psychology and mechanics, just to name a few. Companies make a fortune off many farmers and ranchers on such topics because it’s impossible to study everything and still have a life outside of work.
However, having a basic understanding of each of these topics has the potential to save a producer millions of dollars over his or her lifetime. This is why we are bringing you the “Understanding” series. The series’ purpose is to empower farmers and ranchers by helping them better understand the “why” behind regenerative practices so they can rely more on nature and less on expensive inputs.
Introduction
Part one of this series outlined the basics of soil pH and how management practices can push soil to become more acidic or more alkaline. This article will investigate lime, the universally recommended amendment given to farmers and ranchers working with acidic soil conditions. Understanding the basics of lime will empower farmers and ranchers to make better purchasing decisions and avoid relying solely on the recommendations of someone else. Given these trying economic times, producers need to think long and hard about which products are making money and which are just expensive band-aids.
What is Lime?
Many farmers and ranchers are familiar with agricultural lime, having literally purchased and applied tons of it to their ground to moderate soil pH, as well as other purposes like improving soil structure and balancing fertility levels. In fact, spreading lime is a very old practice that farmers have undertaken for centuries.1 But what exactly is lime and how does it work? Agricultural lime is essentially crushed limestone or chalk. Limestone is a category of rock composed mainly of calcium carbonate (CO32-) that forms in various locations, including deep-sea beds, shallow water and caves. Limestone can be made of many different minerals in combination with CO32- and can be found in many different colors and textures. Magnesium is one such element that is commonly found in limestone alongside calcium. In my home state of Indiana, limestone quarries dot the southern half of the state and provide excellent building material, which is why it’s been used for 35 of 50 state capital buildings, the Empire State building and the Pentagon. In the United Kingdom where I currently live, many fields and pastures have a few inches of topsoil before hitting a deep layer of a specific type of limestone: chalk. The Cliffs of Dover in the Southeast of England provide a good visual to show just how thick these chalk layers can be compared to the tiny layer of soil on top that sustains life.

Chalk is a soft limestone that accumulates in shallow marine environments from the skeletal remains of sea creatures. Just like calcium is an important constituent of our bones, sea creatures like plankton and algae use calcium to make hardened shells. To do this, they absorb free-floating calcium (Ca) and combine it with a compound called carbonate (CO3), a byproduct when carbon dioxide and water combine. The end result is “calcium carbonate” (CaCO3). The image below shows a microscopic view of a certain type of plankton and its intricate shell, which forms a significant part of chalk:

Once creatures like plankton die, they and their shells sink to the bottom and layer on top of each other, which is why you can see very distinct horizontal layers, or “stratification” in the Cliffs of Dover image. This is also why you can see the remnants of little sea creatures when looking at many types of limestone and chalk under a microscope, such as in the thin slice of limestone below:
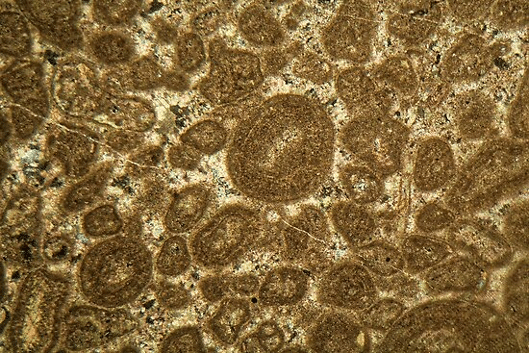
You’ve probably heard the statistic that there is more carbon in the soil than in all the plant life and atmosphere combined. While this is impressive, Earth’s crust is estimated to hold around 75 million (75,000,000!) Pg of carbon in the form of carbonate rock, making it by far the biggest pool of carbon on the planet. By comparison, soil holds a miniscule 3,000-4,000 Pg, vegetation holds 640 Pg and the atmosphere holds 790 Pg. (1 Pg is 1,000,000,000,000,000 g) This means calcium carbonate shells sinking to the ocean floor is a very important part of Earth’s carbon cycle.
Carbon in the rocky reservoir reaches the surface once again in the form of carbon dioxide from volcanic eruptions or as bodies of water dry up and the floor is exposed to the air. An extremely long time passes in either case. That is, unless we humans hit the fast forward button and extract it from the ground, such as what happens when limestone or chalk is mined.
Once mined and ground down, there are two basic types of agricultural lime: dolomitic lime (high calcium and high magnesium) and calcitic lime (high calcium and low magnesium). The difference simply comes down to how much magnesium was in the original limestone or chalk. Just as there are veins of gold and other elements in specific places around the planet, magnesium may or may not be rich in the limestone where it was mined.
Lime is used in both agricultural and industrial processes, including wastewater treatment and sugar beet processing. Farmers and ranchers can often purchase and apply this “spent lime.” Some get it for free. Wood ash is another amendment with the capability of raising pH. In any case, determining the necessity, type and amount of lime is dictated by soil pH, soil fertility levels and cation exchange capacity (CEC).
How Does Lime Change pH?
To briefly review, pH is simply the quantity of hydrogen atoms (H+) hanging around. Very acidic conditions mean lots of H+, while very basic conditions mean very few H+. Lime is applied to neutralize and/or dislodge these H+ from soil particles where they can be leached out of the soil. Lime also dislodges aluminum (Al3+) nutrients that indirectly acidify soil by releasing any H+ it is attached to.
Before lime can work, it needs to fully dissolve in soil water, so the finer the size of the lime, the quicker it will get to work. This is analogous to a large ice cube melting slowly in water versus the same amount of crushed ice cubes melting much quicker. Very fine lime or liquid lime applications can begin working in a little as a few weeks, while coarser lime can take a year to result in an appreciable change in pH. The effects of a typical application last around 3-5 years depending on the fineness and amount.
After lime fully dissolves, 2 H+ are wrangled by oxygen (O) and become part of a harmless water molecule (H2O). Voila! Fewer lone H+. The full reaction results in three products in total, which are water, carbon dioxide and calcium (Ca2+) or Mg (Mg2+). These calcium (Ca2+) and Mg (Mg2+) can bump additional H+ or aluminum (Al3+) off negatively charged soil particles and leave them vulnerable to becoming neutralized or leached deeper in the soil.
As a general rule of thumb, 1 ton per acre of lime will raise pH by 0.3 units on a medium textured mineral soil after 3-5 years. Soils with more clay and/or organic matter require more lime to have the same effect thanks to their higher CEC. (For an explanation of CEC, check out Part 1 of this series.)
Observed Benefits of Lime
Many studies have been undertaken which show the ability of lime to raise soil pH and, consequently, plant productivity. One reason is that a complete range of essential plant nutrients are more available in a pH zone of 6.3–6.8, roughly speaking. In addition, most plant roots are significantly stunted in acidic soils due to excess aluminum (Al3+) availability, which leads to toxicity. Finally, the addition of readily plant-available calcium and magnesium can provide a much-needed shot in the arm to plants that lack access to these crucial nutrients. Magnesium is, after all, the central nutrient in chlorophyll!
Calcium and magnesium are also important nutrients in the soil because they affect the amount of pore space. Calcium is able to clump clay particles together, which creates pore space for air and water, like marbles in a jar. On the other hand, magnesium disperses clay particles and does not allow them to clump tightly. This causes the soil to behave like flour that has water poured on it. Although the particles are fine and spread out, there are not enough large pore spaces between them to let water infiltrate easily. Proper soil function requires zones of soil aggregation surrounded by larger pore spaces for air, water and organisms to move about freely.
This is why many soil scientists and agronomists believe there is an ideal ratio of calcium and magnesium (along with other positively charged nutrients like potassium (K+), sodium (Na+) and even H+) that is necessary for proper soil structure and function. Others, like Dr. Ray Weil of the University of Maryland, disagree with the idea that there are ratios to chase, saying, “This belief is based on a few out-of-date research studies from the mid-20th century and can lead to the wasteful use of soil amendments in an effort to achieve the so-called “ideal” calcium to magnesium ratio.”6
Is Lime Worth It?
Farmers and ranchers are constantly bombarded with conflicting information like the above from experts on every possible topic, so who do we listen to? Unfortunately, it’s no coincidence that most of the results for my Google search on “Benefits of liming soil” took me to web pages of companies that have a financial incentive to sell more lime. That’s just the world we live in and, hey, more power to them. This isn’t always the case, but many times it is. Therefore, I contend, as do my colleagues at Understanding Ag, that you are best served in the long-run by listening to nature.
We recommend doing trials on your own farm or ranch to understand the effect of any product, and lime is no different. This is the gold standard for attaining ROI on your operation. A simple example would be to split a ten-acre plot into thirds: one third receives the recommended rate of lime, one third receives a half rate and one third receives none as a “control” to compare against land that gets lime. Then, observe what happens in subsequent growing seasons. How does pH differ between the test plots? Was there a significant yield drag? Does plant species composition differ? Which plot had the highest net returns? Conduct trials like this on as much land as possible that will allow you to sleep comfortably at night. You’ll be glad you did. The knowledge gained could save hundreds of thousands of dollars over your farming or ranching career.
Arguably more important than running trials is to think about practices that could be acidifying the soil in the first place and brainstorm ways to eliminate or reduce them in your operation. Yes, it’s a natural process in high rainfall areas, but management practices like synthetic nitrogen fertilization, chronic tillage and overgrazing could very well be supercharging acidification, leading to the need for lime every 3-5 years. The fact is that many Understanding Ag consultants have personally reduced or eliminated lime applications in their own operations after making management changes. Many of our clients around the world have done the same.
At the end of the day, agricultural lime is just another tool in the toolbox. It can be seen as a short-term amendment that helps a soil reach a healthy equilibrium or it can be seen as a never-ending, no-questions-asked expense that temporarily raises pH until more is needed. How you view it is up to you.
Next time
Part 3 of this series will discuss alkaline soil management, the opposite issue that many producers in arid regions face. Specifically, we will unpack how various amendments and practices lower pH and whether they are worth the investment.
References
1 https://archive.org/details/onuselimeinagri00johngoog/page/n6/mode/2up
3 https://limetexas.org/lime-public-post/sow-ag-lime-harvest-success/
7 https://environmentalevidencejournal.biomedcentral.com/articles/10.1186/s13750-017-0108-9
8 https://environmentalevidencejournal.biomedcentral.com/articles/10.1186/s13750-017-0108-9
The post Understanding pH Part Two: Examining Lime as a Solution to Acidic Soils appeared first on Understanding Ag.
