INTRODUCTION
Ulcerative colitis (UC) is a chronic disease affecting the large intestine with an ongoing rising incidence worldwide and more recent updated estimates in the United States. Using pooled data from both commercial and public insurance (physician-coded diagnoses), the incidence of UC was estimated to be 6.3 per 100,000 person-years (95% confidence interval [CI], 6.1–6.6) and in adults, higher than that estimated for Crohn’s disease (CD) using the same methodology. The age-standardized, sex-standardized, and insurance-standardized prevalence per 100,000 population is estimated to be 305 (95% CI, 302–308), with a 2020 census extrapolated US prevalence of 1.253 million people living with UC (1).
UC is characterized by chronic inflammation of the large intestine that is frequently associated with involvement of the rectum but often extends proximally to involve additional areas of the colon. Despite advances in understanding environmental associations and risks, the causes of UC remain complex and unknown (2). Absence of rectal involvement has been noted in fewer than 5% of adult patients with UC at diagnosis but may be seen in up to a third of pediatric onset colitis (3). The initial presentation of new UC is usually characterized by symptoms of an inflamed rectum that include bleeding, urgency, and tenesmus (a sense of pressure). The condition may present at any time and at all ages, but there is a predominant age distribution of onset that peaks in the third decade of life. The pattern of inflammatory disease activity is most often, relapsing and remitting, with symptoms of active disease alternating with periods of clinical quiescence (remission). Some patients with UC have persistent disease activity despite available medical therapy, and a small number of patients present with a rapid onset progressive and unresponsive type of fulminant colitis (4,5).
UC causes significant morbidity but fortunately has a low incidence of mortality (6,7). Patients with active disease are more likely to have comorbid psychological conditions of anxiety and depression and are more likely to have impaired social interactions or career progression (8). Longstanding UC is also associated with a defined risk of dysplasia and colorectal cancer (CRC) which is believed to be primarily related to more extensive bowel involvement and longstanding mucosal inflammatory activity (9–11).
Management of UC must involve a prompt and accurate diagnosis, assessment of the patient’s risk for poor outcomes, and early initiation of effective, safe, and tolerable medical therapies. The optimal goal of management is sustained and durable steroid-free remission, accompanied by appropriate psychosocial support, normal health-related quality of life (HRQoL) and social functioning, prevention of morbidity including hospitalization and surgery, and prevention of cancer. To achieve these goals, understanding of the most effective diagnostic, treatment, and preventive strategies is necessary (12). It is now established that a therapeutic means to these ends is the achievement of endoscopic mucosal healing, defined as endoscopic remission (Mayo score of 0 or 1) (13). Furthermore, an evolving principle of management is the concept of disease modification—changing the natural history of the UC toward positive long-term outcomes. As with any medical decision making, involvement of the patients’ preferences forms an important component of care.
The Guideline is structured in sections, each with recommendations, key concept statements, and summaries of the evidence. Over the past 5 years and since the publication of the last guideline from the American College of Gastroenterology (ACG) on this topic (14), the management of UC has grown increasingly complex with availability of additional treatments and therapeutic classes. In addition, approaches to initiate, optimize, and monitor response to existing therapies have undergone considerable evolution. Each recommendation statement has an associated assessment of the quality of evidence and strength of recommendation based on the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) process. Where possible, the GRADE process was used to evaluate the quality of supporting evidence (Table 1). A strong recommendation is made when the benefits or desirable effects of an intervention clearly outweigh the negatives or undesirable effects and/or the result of no action. The term conditional is used when some uncertainty remains regarding the balance of benefits and potential harms, either because of low-quality evidence or because of a suggested balance between desirable and undesirable effects. The quality of the evidence is graded from high to low, where high-quality evidence indicates that the authors are very confident that the true effect lies close to that of the estimate of the effect. Moderate-quality evidence is associated with moderate confidence in the effect estimate, although further research would be likely to have an impact on the confidence of the estimate. Low-quality evidence indicates limited confidence in the estimate, and thus, the true effect could differ from the estimate of the effect. Very low-quality evidence indicates very little confidence in the effect estimate and that the true effect may be substantially different than the estimate of effect (15–17). For this guideline, the authors prioritized direct evidence and did not make recommendations for positioning based on network meta-analyses alone.

Key concepts are statements that are not amenable to the GRADE process, either because of the structure of the statement or because of the available evidence. In some instances, key concepts are based on extrapolation of evidence and/or expert opinion.
This updated UC practice Guideline from the ACG Practice Parameters Committee provides an update to the 2019 publication (14) and to that end is designed with a focus on new approaches and new evidence for treatment and prevention of complications. Additional recommendations regarding preventive care in inflammatory bowel disease (IBD) have been published by the ACG previously and are being updated separately (18). While additional recent guidelines in UC have been published by other societies (19), these ACG guidelines are differentiated by a clinically practical approach to recommendations and prioritization of direct evidence, with less reliance on secondary levels of evidence such as meta-analyses. These guidelines also address some specific concerns and challenges in the US environment. As the number of therapies for UC has increased, so has the evidence. We have added a new section that reviews the evidence and considerations for positioning and sequencing therapies. Prevention of CRC in patients with UC will be addressed in a separate forthcoming document.
Tables 2 and 3 summarize the key concept statements and strength of GRADE recommendations in this guideline.

Summary and strength of GRADED recommendations for the management of ulcerative colitis

Summary of key concept statements for the management of ulcerative colitis
DIAGNOSIS, ASSESSMENT, MONITORING, AND PROGNOSIS OF UC
Recommendations
Key concept statements
Summary of the evidence
Symptoms of bloody diarrhea, presence ofmucous, bowel urgency, tenesmus, and abdominal cramping should trigger consideration of a diagnosis of UC, particularly in the absence of an alternate cause. A full clinical history should include assessment of severity of disease, triggers precipitating onset as well as well as potential alternate etiologies. The primary symptoms assessed include frequency of bowel movements, including number of nocturnal bowel movements, and rectal bleeding as the proportion of bowel movements that are mixed with visible blood. Other important symptoms to assess include bowel urgency, abdominal pain, bowel cramping, and weight loss, which can be a marker of severity of disease. In addition, a thorough history should assess the presence of extraintestinal manifestations including joint pain or swelling, skin eruptions or inflammatory lesions, ocular inflammation, oral manifestations including mouth sores and angular cheilitis, and symptoms suggesting hepatobiliary involvement such as jaundice or pruritus. Potential precipitants of UC may include recent smoking cessation (20), non-steroidal anti-inflammatory drug (NSAID) use (21,22), as well as enteric infections (23). C. difficile infection (CDI) is recognized as complicating a significant proportion of patients with UC and is associated with increased risk of hospitalizations, colectomy, and mortality (24,25). The prevalence of CDI among newly diagnosed or relapsing patients with IBD ranges from 5% to 47% (26). Concomitant CDI with UC has worse outcomes, including higher mortality (27,28). Testing for C. difficile is typically performed by polymerase chain reaction (PCR) or enzyme-linked immunosorbent assay (ELISA) and has been reviewed in recent guidelines (29). Other enteric infections that could mimic UC include infection with Escherichia coli (O157:H7), Salmonella, Shigella, Yersinia, and Campylobacter as well as parasitic infections like amebiasis in the right clinical setting. Therefore, an infectious etiology should always be suspected and excluded at time of diagnosis and in the right clinical setting. Several institutions use comprehensive intestinal pathogen testing through PCR-based assays that include many bacterial and viral pathogens. The prevalence and impact of non-C. difficile intestinal pathogens detected through such assays remain to be robustly established, but the results suggest that C. difficile continues to be the predominant infectious determinant of adverse outcomes in patients with IBD (30).
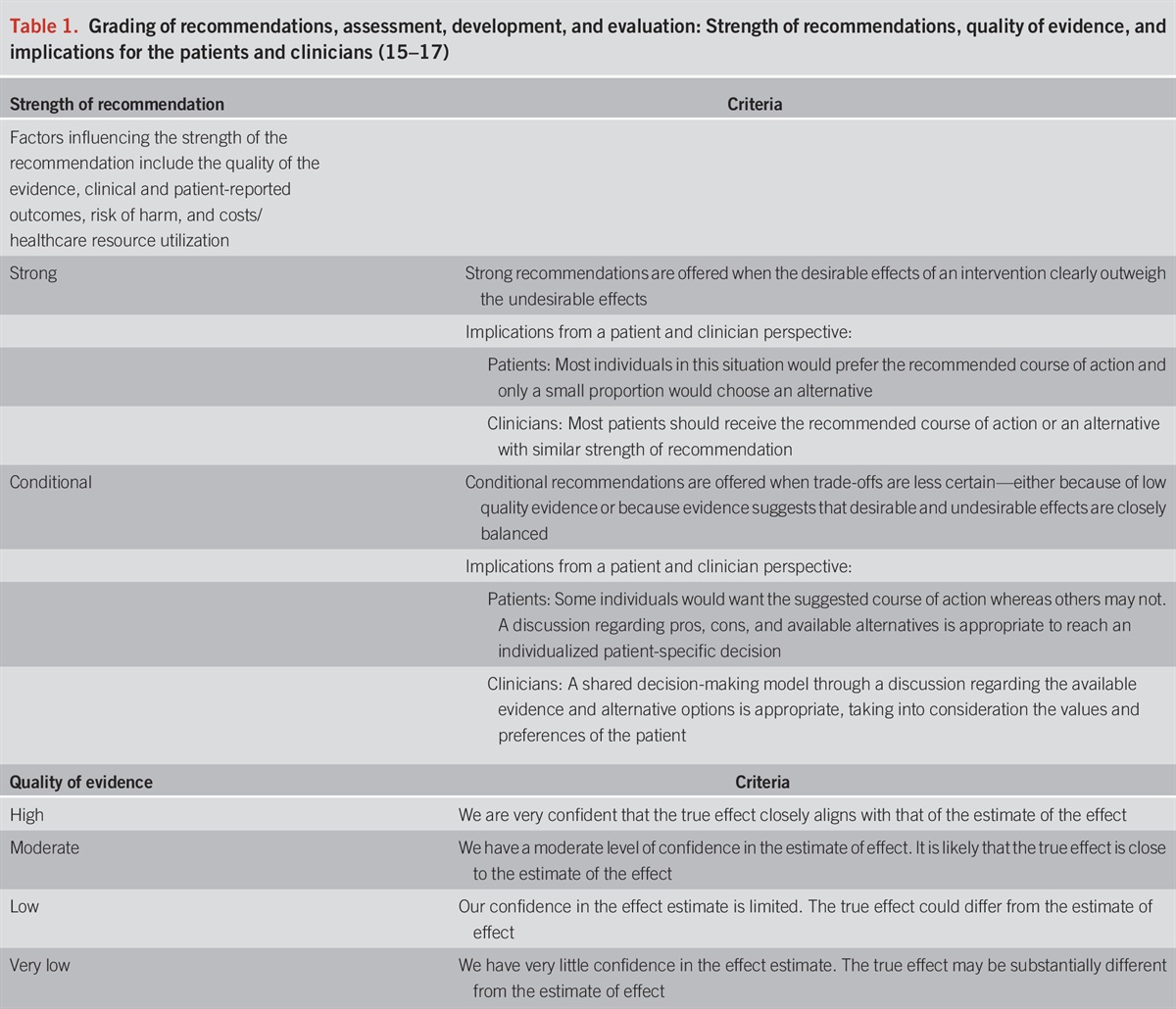
The diagnosis of UC requires a lower gastrointestinal endoscopic examination with histologic confirmation. For most patients, a complete colonoscopy and direct visualization of the terminal ileum should be performed. This allows for assessment of the full extent of disease at diagnosis and can exclude distal ileal involvement, which can be seen with CD. Inspection and description of the ileocecal valve is important, as well, because UC is not associated with an ulcerated or stenotic ileocecal valve. Subsequent endoscopic examinations can then assess response to therapy. However, in individuals with severe disease, a complete colonoscopy may be associated with a greater risk of perforation and in this case a sigmoidoscopy with biopsies is sufficient (31,32). Endoscopically, UC most often presents as a continuously inflamed segment involving the distal rectum and extending proximally. Endoscopic features of inflammation include loss of vascular markings, granularity and friability of the mucosa, erosions, and in the setting of severe inflammation, deep ulcerations and spontaneous bleeding. The index colonoscopy should note involvement of the rectum and complete extent of inflammation. The extent of the disease should be characterized according to the currently used Montreal classification as proctitis (E1, usually defined as ≤15 cm of inflammation), left-sided colitis (E2, defined as more than proctitis, but the extent stops at or distal to the splenic flexure), or extensive colitis (E3, defined as extension proximal to the splenic flexure, with pancolitis defining the entire rectum and colon including the cecum) (Figure 1) (33,34). Proximal histologic extension may be seen even in endoscopically normal-appearing colon and may have implications for defining extent of disease and subsequent surveillance intervals. Therefore, biopsies should be obtained from the proximal endoscopically normal-appearing colon even if the endoscopically inflamed segment seems to be restricted to the distal colon. Similarly, even if the distal rectum seems endoscopically normal, separate biopsies from the rectum should be obtained because patchy histologic inflammation may be seen in 5%–30% of children with UC despite the absence of endoscopically visible inflammation (35). There is a well-defined phenotype of distal UC in which the base of the cecum around the appendiceal orifice is endoscopically and histologically inflamed (36). The pathophysiologic or prognostic implication of this so-called periappendiceal red patch or cecal red patch remains unknown. A separate clinical phenotype of UC has been described in patients with coexisting primary sclerosis cholangitis, in which there is relative or absolute rectal sparing with more active and extensive inflammation in the proximal colon (37). This unique pattern of colitis in the setting of primary sclerosis cholangitis has been postulated to be related to exposure to bile acids and has separately been attributed to the proximal predominance of neoplasia in such patients (38).
Different clinical phenotypes described with ulcerative colitis. Isolated proctitis (a), proctosigmoiditis (b), left-sided colitis (c), extensive colitis (d), and pancolitis (e) are the traditional described phenotypes based on extent of mucosal involvement (historically by barium radiography or endoscopy, more recently defined by histology as well). The primary sclerosing cholangitis phenotype (f) of relative or absolute rectal sparing can be seen in patients and considered a variant of the traditional extent-based phenotypes but is medically managed similarly. The periappendiceal patch or cecal patch phenotype (g) is sometimes seen in patients with limited distal colitis and is similarly managed as well.
Routine upper endoscopic evaluation is not required in adults with a new diagnosis of UC and should be restricted to those who have symptoms of upper gastrointestinal disease. This is different from the pediatric UC population, in which routine upper endoscopy is performed at the time of diagnosis of colitis, and after which, up to 8% of children with UC may have their diagnosis modified to CD based on these upper findings (39,40). In adult patients with UC, gastritis and erosions may be seen in up to a third of patients with UC, and the diagnosis of UC is not modified based on these limited findings, which often are clinically insignificant and seem to resolve over time (41). Imaging the small bowel with a computed tomography (CT) scan or magnetic resonance imaging is also not routinely required in all patients with normal appearance of the terminal ileum on colonoscopy. However, in those with abdominal symptoms not explained by endoscopically active disease, those with suspicion of CD (such as predominantly watery diarrhea, weight loss, or abdominal pain), or those in whom the proximal extent of involvement cannot be evaluated because of severity of inflammation, small bowel imaging may be useful. In particular, when considering surgery in those with UC, a small bowel evaluation may be reasonable to inform surgical management. The utility of intestinal ultrasound in this specific scenario is not yet defined.
Once a diagnosis of UC is made, determining the severity of disease becomes important. We previously proposed new definitions of remission, mildly, moderately, and severely active disease that incorporate both patient-reported outcomes (PROs) and laboratory-based and endoscopy-based values. This ACG UC Disease Activity Index is included and updated in this version of the Guideline (Table 4, Figure 2).


Active UC is frequently marked by an elevation in CRP and erythrocyte sedimentation rate (ESR) (42,43). Although such markers are nonspecific and may be elevated with other causes of systemic inflammation, they often correlate with the endoscopic severity of disease (Table 7) (44). Such markers also have prognostic significance and have a role in predicting risk of colectomy (45–47) and response to therapy (46–48). However, up to a quarter of patients with endoscopically active disease may have a normal CRP and the frequency of elevation is lower in individuals with mild endoscopic activity [reference], so benchmarking the CRP at the time of diagnosis is key to its use and interpretation in later management. Measurement of hemoglobin and serum albumin levels at diagnosis can be helpful in assessing disease severity and prognosis. A low serum albumin is associated with greater risk of hospitalization and surgery and is also associated with reduced likelihood of response to medical therapy (49–52).
FC is a nonspecific neutrophilic marker of inflammation and is elevated in infectious and inflammatory colitis but not in noninflammatory causes of diarrhea such as irritable bowel syndrome. Several studies have confirmed its utility in differentiating IBD from irritable bowel syndrome (IBS) using cut-offs that vary from 6 to 280 mcg/g of stool (53). The pooled sensitivity and specificity of elevated FC for diagnosis of UC are 0.88 and 0.79, respectively, with a modest positive likelihood ratio of 4.2 and a more clinically meaningful negative likelihood ratio of 0.15. In a primary care population, FC in patients with suspected UC (diarrhea, rectal bleeding) can be used to prioritize patients for colonoscopic evaluation, particularly among children (54,55). FC levels are correlated with clinical remission, endoscopic remission, and histologic healing with treatment (56) and are predictive of risk of surgery in acute severe colitis (57). The utility of FC as a marker of inflammation to distinguish IBS from infection and IBD has been recognized and discussed in the ACG Guideline on IBS (58). The utility of FC as a treatment target is further discussed in the management section.
Serologic markers such as perinuclear anti-neutrophil cytoplasmic antibodies (pANCA) may be found in up to 70% of patients with UC, and combination of negative anti-Saccharomyces cerevisiae (ASCA) antibodies with elevated pANCA levels have been proposed to facilitate establishing a diagnosis of UC (59,60). However, the pooled sensitivity of antibody testing for diagnosis of UC is low, and such markers are not used for establishing or ruling out a diagnosis of UC. While pANCA positivity has also been associated with treatment refractory UC, the evidence supporting this is limited and there is currently no role for such testing to determine the likelihood of disease evolution and prognosis (61,62).
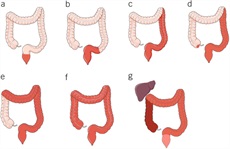
In addition to extent, determination of activity and severity of disease is important to select the appropriate treatment algorithm. Commonly, severity of UC has been classified according to the Truelove and Witts criteria published in 1955 (63). Mild colitis is defined as fewer than 4 bowel movements daily, normal temperature, heart rate, hemoglobin (>11 g/dL), and ESR (64) including the Mayo score (Table 5) (65), Seo Index (66), Rachmilewitz Index (67), Simple Clinical Colitis Activity Index (SCCAI) (Table 6) (68), PRO2 (69), and the pediatric UC activity index (70). Although disease extent broadly affects prognosis, it should not limit therapeutic options. While most clinical activity indices have not been rigorously validated, there is broad agreement between most of the indices (71) and they generally correlate well with endoscopic disease activity. In a prospective comparison, the pediatric UC activity index, SCCAI, and partial Mayo score demonstrated the best validity and responsiveness (70,72,73). The PRO2 of stool frequency and rectal bleeding (derived from components of the Mayo score) has been shown to discriminate between active drug and placebo and yielded similar effect sizes for remission when applied to previously collected clinical trial data. This has been proposed as an interim outcome measure when combined with endoscopic data (69). In addition, a Modified Mayo Score which excludes the subjective Physician’s Global Assessment is now used as a standard endpoint in clinical trials. More recently, bowel urgency has been developed and validated as a substantial PRO in patients with UC and has been reported to be one of the most bothersome and disruptive symptoms, distinct from stool frequency and bleeding (74). In a multinational survey of patients and clinicians, bowel urgency was rated by patients to be the second most common symptom and was noted to be more bothersome than rectal bleeding. Notably, clinicians did not rate bowel urgency at this level of significance (6). Urgency may be reported using a validated 11-point numeric rating scale (74), a PRO-UC diary (75), or the UC-PRO signs and symptoms diary (76), but in clinical practice, the routine assessment of urgency in a more binary or quartile (always, sometimes, rarely, never) should be considered and may be more practicable.
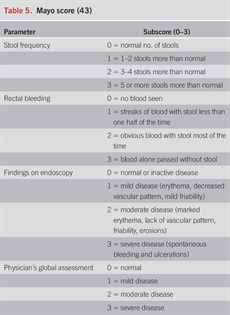
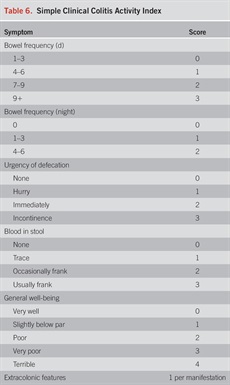
Simple Clinical Colitis Activity Index

Prior definitions of disease severity have been used in clinical trials, but not in clinical practice. Inclusion criteria for clinical trials of agents for moderately to severely active UC have required components such as (i) inability to taper off prednisone, (ii) prior failure of immunosuppressants, and (iii) moderate-to-severe disease defined by Modified Mayo score (including the specific endoscopy subscore). In clinical trials, the definition of clinical remission has been a MES of 0 or 1, stool frequency of ≤1, and absence of rectal bleeding. In clinical practice, the previously used definitions of remission refer to clinical parameters of current relapse (number of bowel movements, bleeding, urgency, evidence of toxicity such as vital signs or colonic dilation), but do not include objective parameters of increased disease activity other than CRP (which lacks sensitivity and specificity). These measures also do not place the current relapse in the context of the prior disease course as our prior and this updated guideline recommends. In addition, when using a newer disease activity definition that takes into account disease course, any patient with more than mildly active disease should be treated according to recommendations for moderately to severely active UC.
In the absence of endoscopy, other objective markers of inflammation can be considered such as normalization of FC and CRP. These markers of inflammation have independent value for assessing inflammation (or lack thereof) but may also be helpful in follow-up if baseline levels are benchmarked to the original diagnostic endoscopy. More recent measures of remission now include symptomatic remission (no rectal bleeding, normal stool frequency, and no urgency) and endoscopic evidence of mucosal healing (a MES of ≤1). Retrospective data have investigated histologic remission as a potential therapeutic target and have shown histologic quiescence and histologic normalization to be predictive of relapse-free survival (77). An ongoing prospective randomized trial of treatment endpoints in UC treatment with vedolizumab has demonstrated a 34% success rate of achieving week 16 symptomatic, endoscopic, and histologic disease control free of steroids (defined as disease clearance) (78,79). However, available data do not yet support histologic healing or normalization as a goal of treatment for patients with UC.
With increasing recognition of endoscopic mucosal response and remission as treatment targets and their prognostic significance for future relapses, need for hospitalization, and surgery, it is essential to include endoscopic severity assessment in the diagnosis and management of UC (52,80). There are several tools to quantify endoscopic activity in UC, although few have been rigorously validated (81). The MES is frequently used in clinical trials and is simple to use in clinical practice, ranging from 0 for normal or inactive disease to 3 for severely active disease (65). Table 4 summarizes the different parameters used in this guideline for the purpose of defining mildly active and moderately to severely active UC (63,82). Figure 2 shows representative endoscopic photographs describing the most commonly used index in clinical practice, the MES. There is active innovation and research into artificial intelligence approaches to automated or assisted endoscopic or histologic activity assessments. Such approaches promise to provide more efficient and reliable endoscopic examinations, enable rapid screening and eligibility assessment for clinical trials, and yield new insights into the pathophysiology of UC (83–85). Despite the promise of such an approach, this is not yet recommended for clinical practice.
New for these updated guidelines is a defined role for intestinal ultrasound (IUS) as a tool to measure UC disease activity and monitor response to therapy or disease relapse. IUS is a point-of-care test that involves a high frequency transducer and transabdominal approach to measuring bowel wall thickness, color flow Doppler (hyperemia), and other parameters as measures of active and chronic colitis. The transabdominal approach is not sufficient to see the rectum, so a transperineal approach can be used (86). In a systematic review and meta-analysis of 16 studies in IBD comparing IUS with endoscopy and with biochemical markers, IUS had high pooled sensitivity, at 85% (95% CI, 78%–91%), and specificity, at 92% (95% CI, 86–96 (87)). IUS is of low yield for endoscopically mild UC, but in moderately to severely active UC, moderate wall thickening (>3 mm) is found, along with submucosal edema and hyperperfusion. IUS can detect response to therapy as soon as 2 weeks (88). The use of this noninvasive imaging modality has provided new insights into the rapidity of treatment response in UC. There is an established training pathway and credentialing for acquisition of IUS skills (89,90).
UC is also associated with psychosocial and economic disruption and disability. There are ongoing efforts to quantify such disability though validated indices that correlate well with disease severity and quality of life (82,91,92). There are insufficient data to recommend routine use of such scores in clinical practice. However, it is important to include assessments of the impact of the disease on the patients’ lives in the determination of overall severity and selection of the appropriate treatment algorithm (8).
Evaluation of UC during relapses should include assessment of severity of symptoms and potential triggers, including enteric infections (particularly C. difficile), NSAID use, and recent smoking cessation. Nonadherence to therapy is common in patients with UC and is associated with increased risk of relapse and cost of care (93,94). In addition to symptomatic assessment, objective measures of disease activity should accompany evaluation of suspected relapse. This may include repeat FC, CRP, endoscopic evaluation, or IUS. UC is an evolving disease, and the risk of disease extension should be kept in mind in individuals with initially localized disease, particularly with nonresponse to topical treatment. Up to 46% of patients with proctitis and 70% with left-sided colitis may develop extensive colitis on follow-up (95). It is important to recognize that endoscopic evaluation in individuals with loss of response may reveal patchiness of endoscopic and histologic activity including an appearance of relative rectal sparing with use of topical treatments.
A comprehensive assessment of severity of UC should include predictors of an aggressive disease course, need for colectomy, and response to therapies. Several prospective cohorts have examined the role of clinical parameters, genetics, and serologic markers in predicting need for colectomy in UC, but only clinical parameters are recommended currently (96,97). Extensive colitis, deep ulcers by endoscopy, need for systemic steroids, young age at diagnosis (younger than 30 years), and elevated CRP or ESR are associated with higher rates of colectomy (96,98). Patients with a prior hospitalization for their UC are also at a higher risk for subsequent colectomy (99). The yield of genetic or serologic markers in predicting severity and course of UC has been modest at best, and their use cannot be recommended in routine clinical practice based on available data (61,62). Table 8 summarizes the factors associated with increased risk of colectomy and a poor prognosis (5).
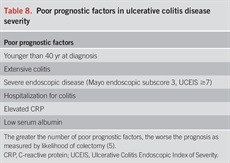
Poor prognostic factors in ulcerative colitis disease severity
GOALS FOR MANAGING PATIENTS WITH UC
Recommendations
Key concept statements
Summary of the evidence
Patients’ and providers’ goals may not always align. Studies have identified disparities between HRQoL measures as perceived by patients and their providers (6,101,102). Symptoms alone should not be used as the only measure of remission, and patients need to be educated about these concepts because symptomatic remission can lag behind healing (103). In addition, a large portion of patients with UC have mucosal inflammation without clinical symptoms (104). Therefore, it is important to rely on objective clinical targets and use validated scores and instruments (including endoscopy) in confirming remission (105,106). According to US Food and Drug Administration (FDA) guidance, a PRO involves the generation of items from qualitative patient interviews and testing for reliability and responsiveness to changes in clinical health (107). An optimized PRO derived from the Mayo score and the SCCAI has been validated (108). Resolution of rectal bleeding and bowel urgency, normalization of bowel habits, and improvement in general well-being should be the goal for patient-reported symptoms.
Disease activity indices used in clinical trials can be used to define steroid-free remission. These include the Mayo Score (65) (and Modified Mayo Score), Rachmilewitz (67), SCCAI (68), and pediatric UC activity index (PUCAI) (73). Targets have been defined for the treatment of UC, and goals of therapy should be directed at these targets. A treat-to-target approach focuses on HRQoL as a primary goal achieved through serial assessment of disease activity by using objective and clinical biological outcome measures and a shared decision-making approach to subsequent adjustment of treatments (109).
Therapeutic targets have been recommended for UC, as part of the second Selecting Therapeutic Targets in Inflammatory Bowel Diseases (STRIDE II) consensus statement, which was based on a systematic literature review and expert opinion of 20 IBD specialists and the International Organization for the Study of IBD. The targets for UC were composite endpoints that include resolution of rectal bleeding, normalization of bowel habits, and a MES of 0 or 1. STRIDE II proposed that these endpoints should be assessed at a minimum every 3 months during the active phase of disease (110,111). The STRIDE II recommendations set endoscopic remission as a primary target was based on evidence that supports that the degree of mucosal healing is correlated with clinical outcomes, including avoiding colectomy (52,80). STRIDE II also acknowledged that FC and CRP may be used as surrogates for inflammation. It is acknowledged that endoscopic improvement (MES of 0 or 1) rather than complete healing (MES of 0) may be sufficient and associated with similar outcomes (52).
At the time of the STRIDE II, histology was not yet identified as a target for treatment, but the research and understanding of this biomarker has evolved. Recent studies and critical reviews of histology as a marker of disease activity and potential endpoint of therapy demonstrate that the presence of active microscopic inflammation (defined by the presence of mucosal neutrophils) is predictive of clinical relapse, hospitalization, and steroid use (112). Conversely, the absence of histological inflammation is associated with stable remission and reduced need for steroids (13). In addition, there are several significant studies which demonstrate that increased degree of histological inflammation is associated with dysplasia and CRC (also discussed below) (10,113,114). However, many patients with endoscopic improvement will have histologic activity, and the benefits of intensifying or changing therapy in that situation has not been demonstrated. Thus, at this time, histologic activity carries prognostic value but is not a required treatment target. Transmural inflammation has been described in UC, but transmural healing is not yet a target of treatment (115).
Calprotectin is an antimicrobial manganese sequestration protein complex which comprises 60% of the soluble proteins in the cytosol of neutrophils (116). It is secreted by an unknown mechanism during inflammation, is a stable protein in stool, and quantification of it is possible with commercially available laboratory assays. FC levels correlate with degrees of endoscopic and histologic inflammation in UC and therefore have been proposed as a marker of disease activity to guide treatment (116,117). FC levels are more sensitive and specific than serum inflammatory markers and obviously also less invasive than endoscopy or mucosal biopsies, so this assessment has become routine for many clinicians who are managing patients with UC (53,118). FC therefore has been proposed as a monitoring tool to assess response to therapy or subclinical relapse (119,120). Higher levels of FC correlate with more endoscopically severe disease, but absolute levels may not correlate with the colonic extent of inflammation. The cut-offs for defining clinical or endoscopic remission and as the optimal therapeutic target have not been studied prospectively and are thus not amenable to the GRADE process. Relevant cut-offs will differ based on whether studies of FC are assessing (i) mucosal healing (by endoscopy or histology) or (ii) clinical relapse and are limited by intra-patient variability (121–123). In separate studies, FC 121) and mucosal healing (122), respectively, while a FC > 321 mg/g in clinical remission predicted an increased risk of relapse at 6 and 12 months (124). As with other inflammatory markers, the degree of elevation of FC correlates with burden of inflammation and values may be normal or borderline in mild disease and may need to be repeated over time. A meta-analysis of 25 eligible studies revealed that FC had a pooled sensitivity for endoscopic inflammation in UC of 87.3% with a specificity of 77.1% and area under the curve of 0.91 (125). This analysis described that the optimum cut-off varied widely by studies, but that the best sensitivity of 90% (87.9–92.9) was achieved at a cut-off level of 50 μg/g, whereas the best specificity of 78.2% (75.7–80.6) was achieved for cut-off levels greater than 100 μg/g (125). There are a number of other clinical factors that are associated with increased FC levels (e.g., infections and NSAIDs). Mildly elevated FC may be seen with proton pump inhibitors and obesity (126). In an individual patient, serial FC can be useful as a predictor of response to therapy or relapse. This principle has been demonstrated in multiple clinical trials of therapies for UC (117,127–129). FC levels have been correlated with histologic disease activity as well (130). Thus, it is no longer considered an experimental biomarker; the robust available data support FC as an appropriate surrogate to sigmoidoscopy or colonoscopy for assessment and monitoring of mucosal inflammation.
Therapeutic management of UC
Therapeutic management in UC should be guided by the extent of bowel involvement, an assessment of disease activity (i.e., quiescent, mild, moderate, or severe), and disease prognosis. This updated guideline emphasizes that patients with moderately to severely active UC or those who have UC with high risk of hospitalization or colectomy should be treated with therapies that have evidence for their efficacy in this degree of active disease or with this specific prognosis, based on evidence in clinical trials and real world observational studies. We recommend that prognosis should guide choice of therapy as much as activity of inflammation at the time of acute illness.
MANAGEMENT OF MILDLY TO MODERATELY ACTIVE UC
Induction and maintenance of remission in mildly to moderately active UC
Recommendations
Key concept statements
Summary of the evidence.
A meta-analysis of 11 randomized controlled trials (RCTs) of patients with UC treated with 5-ASA for induction or maintenance demonstrated superiority of 5-ASAs in inducing remission compared with placebo (131). In this analysis, patients receiving 5-ASA were more likely to achieve remission. Only 60.3% of patients treated with 5-ASAs failed to reach remission, compared with 80.2% of patients treated with placebo (relative risk [RR] = 0.79, CI 0.73–85; P = 0.009, number needed to treat [NNT] = 6). Efficacy of 5-ASAs in inducing remission was similar whether remission was defined clinically or endoscopically. Another meta-analysis of 38 studies in patients with mildly to moderately active proctitis or left-sided UC found that rectal 5-ASA was superior to placebo, with a pooled odds ratio (OR) of 8.30 (95% CI 4.28–16.12; P P 132). Rectal 5-ASA was also found to be superior to rectal corticosteroids for inducing symptomatic remission (OR = 1.65; 95% CI 1.1–2.5) (132). Despite the superiority of rectal 5-ASA over rectal steroids, steroids remain an important option for patients with mildly active left-sided UC who cannot retain rectal 5-ASA, have hypersensitivity to 5-ASA, or who are not responding to 5-ASA (132).
In left-sided UC, a meta-analysis of 4 RCTs using combination treatment with rectal 5-ASA enemas (1 g/d) combined with oral 5-aminosalicylate (at least 2.0 g/d) was more effective than oral 5-ASA alone for induction of remission (relative risk induction failure RR = 0.65; 95% CI 0.47–0.91) (133). Another meta-analysis comparing the 2 regimens showed a RR of 0.86 for induction failure when using the combination therapy (95% CI 0.81–0.91) (134). However, in patients with mildly active extensive colitis, oral 5-ASA at a dose of at least 2.0 g daily is preferred to induce remission (134,135). In a subsequent meta-analysis, a low dose of 2.0–2.4 g of 5-ASA was found to be just as effective as a higher dose (4.8 g) (RR = 0.91; 95% CI 0.85–0.98) (134). A subgroup analysis indicated that patients with more active (moderate) disease may benefit from the higher dose of 4.8 g/d (136). Once daily dosing of oral 5-ASA was demonstrated to be as effective as multiple doses daily and may facilitate compliance (136).
Treatment with 5-ASA therapy has been shown to be efficacious and safe as monotherapy for induction of moderately but not severely active UC. One meta-analysis showed that patients with moderately active disease benefited from treatment with 2.4 g/d, while corticosteroid therapy remained more effective for patients with severe disease (134). A lack of response to 5-ASA should prompt consideration that a patient has moderate-to-severe UC and treatments for that type of colitis should be initiated. In addition, diarrhea as an adverse event of 5-ASA therapies should be considered. A rare paradoxical increase in diarrhea associated with 5-ASA has been described in a subset of patients (137,138).
In patients with mildly to moderately active UC who fail to reach remission with appropriately dosed 5-ASA, switching to an alternate 5-ASA formulation is not recommended because meta analyses have not demonstrated a therapeutic difference between different formulations (139,140). However, no formal switch studies have been published. Nonetheless, clinicians should be aware that the approved dose of some mesalamine preparations in maintenance is lower than the recommended effective doses for induction. Specifically, a specific extended release mesalamine capsule formulation available in the United States is approved for maintenance at a dose of 1.5 g/d but not for induction (Apriso; Salix Pharmaceuticals, Bridgewater, NJ) so may not provide optimal dosing for successful induction of remission. In patients with UC who fail to respond to appropriate doses of oral 5-ASA therapy, oral corticosteroids can be used to induce remission. A meta-analysis showed that corticosteroids are more effective than placebo for induction of remission (RR = 0.65; 95% CI 0.45–0.93) (141). The typical starting doses of oral prednisone are 40–60 mg daily, usually in a single dose, and clinical response is expected within 5–7 days of treatment. There were no observed differences however when starting at doses higher than 60 mg/d (142). The duration of systemic corticosteroids should be as short as possible with early initiation of steroid-sparing therapy. The speed of the taper should be guided by clinical symptoms, cumulative steroid exposure, and onset of action of alternate therapies.
Budesonide is a locally acting corticosteroid with high first pass metabolism and minimal systemic side effects. In patients with UC who fail to respond to 5-ASA, budesonide MMX 9 mg for 8 weeks was found to be superior at achieving a combined endpoint of clinical and endoscopic remission compared with continuing 5-ASA and placebo (P = 0.049) (143). The use of corticosteroid preparations with high first-pass metabolism and low systemic effects may be preferred over systemically active glucocorticoids. Oral budesonide MMX is also safe and more effective than placebo in inducing remission in patients with mildly active UC. In a prospective RCT, patients given 9 or 6 mg budesonide MMX or mesalamine achieved clinical remission 17.9%, 13.2%, and 12.1% of the time, respectively, in comparison with 7.4% in the placebo group (P = 0.0143, P = 0.1393, and P = 0.2200) (144). A Cochrane systematic review and analysis of the efficacy and safety of oral budesonide for the induction of remission in UC identified 6 studies including 1808 participants who received budesonide-MMX. A subgroup analysis by concurrent mesalamine in patients (n-442) who were not 5-ASA resistant identified higher efficacy compared with those who were 5-ASA refractory (RR 2.89, 95% CI 1.59–5.25; 442 patients), and additional analysis identified that budesonide was most effective in patients with left-sided UC (RR 2.98, 95% CI 1.56–5.67; 289 patients). The overall analysis concluded that budesonide was safe in UC and did not lead to adrenal suppression compared with placebo (145).
Adherence to medication is a factor in relapse in patients with mildly active UC. A meta-analysis of 3 trials found no significant differences in efficacy or adherence between once-daily and conventionally dosed 5-ASA for induction of remission in patients with UC (nonremission RR, 0.95; 95% CI, 0.82–1.10) (134,146). However, clinical trial populations are known to have higher adherence rates than clinical practice settings. Prevalence of nonadherence in the community is high (40%), reaching up to 68% in patients on more than 4 prescription medications (147). An RCT found that patients with proctosigmoiditis preferred once-daily mesalamine dosing over 3 times daily dosing. Patients also had a significantly higher rate of clinical remission in the once-daily dose group (86%; n = 97) vs the 3 times a day (TID) group (73%; n = 100; P = 0.0298) (148). Therefore, reinforcement of adherence is an important aspect of management of UC, and any means to optimize adherence should be used, including discussing once-daily dosing options with patients given these data on similar efficacy and safety.
In patients with mildly to moderately active UC, on appropriately dosed 5-ASA, an 8-strain probiotic has been studied as an adjunct to 5-ASA therapy to improve symptoms, as compared with no treatment. In a meta-analysis from 2017 including 22 studies of probiotics in the treatment of IBD, there was no benefit of probiotics in general for induction of remission. However, when only studies of this probiotic were included (n = 3), there did seem to be a benefit (RR 0.74 95% CI 0.63–0.87) in these small studies. All these studies were at risk of bias, and the quality of the evidence was too low to make a recommendation for or against the use of the 8-strain probiotic as an add-on therapy in UC (149). In one clinical trial using this probiotic as add-on therapy to 5-ASA, endoscopic improvement was not achieved (150). A meta-analysis of 3 studies found that treatment with E. coli Nissle 1917 was comparable with mesalamine therapy in patients with inactive UC (RR pooled 1.08, 95% CI 0.86–1.37) (151). Similar methodological concerns for these studies exist, including small sample size, risk of bias, and high degree of heterogeneity, limiting the level of evidence supporting this intervention. The control population included placebo or mesalamine. However, the comparison doses of mesalamine were often ≤1,500 mg (less than a recommended maintenance dose). In one clinical trial, patients with UC randomized to E. coli Nissle were less likely to reach remission as compared with those on placebo (152). Therefore, there is not sufficient evidence to recommend E coli Nissle for induction of remission of UC. Given the available evidence and safety of other therapeutic options and the absence of robust, generalizable evidence for any specific probiotic for induction of remission, we recommend against monotherapy with any probiotic to induce remission in patients with mildly to moderately active UC.
Similarly, fecal microbiota transplantation (FMT) has showed some promising data in the treatment of UC and has been studied in 3 RCTs (153–155). These trials of FMT in UC have different designs, delivery mechanisms, donor types, and inclusion criteria. The RCTs for FMT have had variable benefits, but not significant steroid-sparing effects. The variability in fecal donors, delivery systems, duration of treatment, and endpoints make interpretation of these results difficult, and this is not currently a recommended treatment option for UC (156). The subsequent stringent testing and ongoing scrutiny of FMT safety further limits this treatment option for patients with UC.
A meta-analysis of 7 trials assessed the efficacy of topical mesalamine in preventing relapse in controlled UC (135). Only one of the included placebo-controlled trials assessed patients with extensive UC, while the remaining trials recruited patients with proctitis, proctosigmoiditis, or left-sided colitis. Among the trials that reported disease duration, the mean duration was 5–7 years. Compared with placebo, patients receiving topical mesalamine had a RR of 0.60 (95% CI 0.49–0.73) for relapse. Two trials evaluated time to relapse in patients with rectal disease, and both found that patients receiving topical mesalamine experienced relapse at a later time as compared with placebo. Corticosteroids are ineffective in maintaining remission and are limited by their side effects and possible complications. Therefore, corticosteroids are not used for maintenance of remission (157–160).
A meta-analysis of 11 trials demonstrated the efficacy of oral 5-ASA agents (mesalamine, olsalazine, and sulfasalazine) compared with placebo in patients with quiescent UC (distal, left-sided, or extensive colitis) in maintenance of remission (131). The overall RR of relapse was 0.65 (95% CI 0.55–0.76). Fewer patients on the high-to-standard dose of 5-ASA (≥2 g) experienced relapse of their quiescent disease compared with those on low dose (140). However, sulfasalazine is often limited by intolerance (headache, nausea), allergy to the sulfa moiety, and need for multiple daily doses. A separate extended-release capsule of mesalamine granules was studied in a randomized, placebo-controlled trial in patients with mild-to-moderate UC who were in remission (defined by the Sutherland Index and endoscopy). A significantly greater percentage of patients receiving mesalamine granules 1.5 g/d compared with placebo were in remission at 6 months (79.9% vs 66.7%; P = 0.03) (161).
MANAGEMENT OF MODERATELY TO SEVERELY ACTIVE ULCERATIVE COLITIS
Induction of remission in moderately to severely active UC
Recommendations
Key concept statements
- 5-ASA therapy could be used as monotherapy for induction of moderately but not severely active UC.
- In patients with moderately active UC, consider nonsystemic corticosteroids such as budesonide MMX before the use of systemic steroid therapy.
- In patients with severely active UC, consider systemic corticosteroids rather than topical corticosteroids.
- Corticosteroids may be avoided entirely when other effective induction strategies are planned.
MAINTENANCE OF REMISSION IN PATIENTS WITH PREVIOUSLY MODERATELY TO SEVERELY ACTIVE UC
Recommendations
Key concept statements
Summary of the evidence
Systemic corticosteroids are an acknowledged induction strategy for moderately to severely active UC, with several small controlled studies demonstrating benefit to this strategy (135,141,163). In a meta-analysis of trials in patients with active UC, the use of systemic glucocorticoids compared with placebo demonstrated a benefit favoring steroids (RR of failure to achieve remission = 0.65; 95% CI 0.45–0.93) (141,164). A colonic delivery system of budesonide offers more directed therapy fewer systemic side effects, given the high first-pass hepatic metabolism of budesonide. In a dose-finding RCT in mildly to moderately active UC, patients receiving oral 9 mg budesonide MMX were more likely to achieve induction of combined clinical and endoscopic remission at week 8 compared with placebo (OR = 2.71; 95% CI 1.19–6.16) (135). A multicenter phase III RCT showed similar results, with significantly more patients treated with budesonide MMX 9 mg (but not 6 mg) achieving combined clinical and endoscopic remission at week 8 compared with placebo (OR = 4.49; 95% CI 1.47–13.72; P = 0.0047) (163). Patients receiving budesonide had a similar rate of adverse events when compared with placebo (163). Although corticosteroids are efficacious in inducing remission in patients with active UC, they should not be used for maintenance of remission and should be tapered instead (158–160). While the optimal tapering regimen has not been determined, the dose is usually reduced over 8–12 weeks (157). Newer therapies for UC have demonstrated impressive steroid-sparing or even steroid-avoidance results, suggesting that when treatments for moderately to severely active UC are going to be prescribed, steroids might be avoided altogether in favor of the induction potency of the primary treatment planned.
A patient with UC who needs corticosteroids (as opposed to the one who is prescribed them with 5-ASA and without allowing the 5-ASA to be tried as monotherapy) should be treated with therapies approved for moderately to severely active UC and that have demonstrated steroid-sparing effects. This movement from a single steroid exposure to more effective treatment strategies will reduce morbidity from ongoing active UC and prevent complications from excessive steroid exposure.
Thiopurines are slow-acting and do not induce remission in moderately to severely active UC (164–166). Similarly, methotrexate is not an effective induction agent in moderately to severely active UC. Prior studies of oral methotrexate have not demonstrated benefit, and 2 meta-analyses of methotrexate 25 mg intramuscularly are negative (165,167). In the European multicenter study of methotrexate for induction of remission of moderately to severely active UC, a higher proportion of patients receiving parenteral methotrexate (25 mg/wk) achieved steroid-free remission at week 16, but this result did not achieve statistical significance (168).
In 2 RCTs, thiopurines have not been shown to provide significant maintenance benefit in patients with UC who have had induction of remission with corticosteroids (RR = 0.85; 95% CI 0.71–1.01) (165). In an additional 3 RCTs, azathioprine prevented relapse in 127 patients (RR = 0.6; 95% CI 0.37–0.95) (165). Another systematic review encompassing 1,632 patients with UC in 30 studies showed that azathioprine and mercaptopurine had a 76% mean efficacy in maintenance of remission. When compared with placebo, treatment with thiopurines resulted in an absolute risk reduction of 23% and an NNT of 5 to prevent recurrence (OR = 2.59; 95% CI 1.26–5.3) (169). Thiopurine therapy also provided clinical benefit when treating patients who had failed or could not tolerate mesalamine or sulfasalazine (170). On the other hand, in a prospective RCT, methotrexate was not found to be superior for maintenance of remission when compared with placebo (171). The US-based MERIT (Methotrexate Response in Treatment of Ulcerative Colitis)-UC trial demonstrated that parenteral methotrexate (25 mg/w) was not superior to placebo in maintaining remission after steroid induction (172). In this study, 29/44 (66%) patients receiving methotrexate experienced relapse compared with 25/40 (63%) patients receiving placebo (172).
The small molecule S1P receptor modulators ozanimod and etrasimod have a mechanism of action that sequesters activated lymphocytes in lymph nodes in patients with UC and results in decreased cellular inflammation in the bowel and a corresponding decreased circulating lymphocyte count. Ozanimod is an oral S1P receptor antagonist for subtypes 1 and 5. In a Phase 3 RCT involving patients with moderately to severely active UC, patients received either 1 mg ozanimod hydrochloride (equivalent to 0.92 mg of ozanimod) or a placebo for 10 weeks. A second group received open-label ozanimod for the same duration. At the end of 10 weeks, a larger proportion of patients receiving ozanimod achieved clinical remission (18.4%) compared with placebo (6.0%, P P P P 322). In post hoc analyses, patients who were naive to advanced therapy were more likely to achieve remission than those who were advanced therapy (mostly anti-TNF) exposed, and those who had moderately active disease were more likely to respond to ozanimod than those who had severely active disease (173). These subset analyses suggest that ozanimod should be used earlier in the treatment algorithm of moderately to severely active UC. The overall rate of adverse events was higher with ozanimod than placebo, but serious adverse events were similarly rare in both groups. There was a higher incidence of transient and asymptomatic bradycardia and transient abnormal transaminases in the ozanimod group (174). Significant cardiovascular disease, severe untreated sleep apnea, cardiac conduction defects, and concomitant use of monoamine oxidase inhibitors contraindicate the use of ozanimod. Etrasimod is a S1P receptor modulator that activates S1P receptor subtypes 1, 4, and 5. The efficacy of etrasimod in moderately to severely active UC was examined in the ELEVATE (Etrasimod as Induction and Maintenance Therapy for Ulcerative Colitis Trial) UC 52 and ELEVATE UC 12 studies. In the induction trials, patients received etrasimod 2 mg daily orally or placebo for 12 weeks. Using a treat-through design, during the maintenance phase, patients continue the same dose for an additional 40 weeks of treatment. In the ELEVATE UC 52 study, at the completion of 12 and 52 weeks of treatment, 27% and 32% of patients receiving etrasimod achieved clinical remission compared with 7% and 7% of patients treated with placebo (P P P = 0.026) and endoscopic improvement (31% vs 19%, P = 0.0092) at week 12 compared with placebo. Unlike all other modern pivotal trials for moderately to severely active UC which excluded patients with isolated proctitis, the pivotal trials for etrasimod included patients with isolated proctitis and demonstrated a clinical remission rate in these patients of 43.2% at week 12, compared with 13.6% with placebo (P 175).
Tacrolimus enemas or suppositories are effective for treatment of refractory distal colitis. In a double-blind RCT conducted in 85 patients with refractory ulcerative proctitis, treatment with once daily 2 mg tacrolimus suppositories or 3 mg daily beclomethasone suppositories was associated with similar rates of clinical response at 4 weeks (63% and 59%, respectively, P = 0.812) (176). Similar side effects to cyclosporine may be observed, but these are less common given the low dose, topical delivery system, and low systemic absorption (176–178).
The data for topical tacrolimus and oral etrasimod in moderately to severely active ulcerative proctitis reinforce a general principle that a patient with moderately to severely active disease regardless of the extent of bowel involvement should be treated with therapies that have demonstrated efficacy for the activity and severity of the disease.
Infliximab, adalimumab, and golimumab are effective for the induction of remission of moderately to severely active UC (103,179–181). All 3 anti-TNF agents have demonstrated superiority over placebo in achieving the primary endpoints of response and remission, but there have been no head-to-head trials comparing the agents with one another (182–184). However, there is considerable evidence specifically for infliximab in UC which demonstrates a precise and strong benefit in UC (185). In addition, in patients with moderately to severely active UC who have responded to anti-TNF therapy during induction dosing, anti-TNF agents are superior to placebo in maintaining remission (182). A systematic review and meta-analysis including 6 placebo-controlled, double-blind studies demonstrated that adalimumab, golimumab, and infliximab were all more efficacious than placebo in maintaining clinical remission in patients with UC (183).
The alone or in combination with thiopurines (ACT 1) and ACT 2 trials were the first large double-blind placebo-controlled studies to examine anti-TNF therapy in moderately to severely active UC. In these 2 similarly designed trials, 728 patients with moderately to severely active UC in whom conventional therapy failed with glucocorticoids ACT 1 or glucocorticoids alone or in combination with thiopurines and 5-ASAs (ACT 2) were randomized to placebo. Infliximab was infused at doses of 5 mg/kg or 10 mg/kg at weeks 0 and 2 and then every 8 weeks through week 46 (ACT 1) or week 22 (ACT 2). In ACT 1 at week 8, 69% and 61% of patients receiving infliximab at 5 mg/kg and 10 mg/kg, respectively, had a clinical response, compared with 37% of patients receiving placebo (P P P P 103). In these studies, infliximab also achieved steroid-sparing and mucosal healing properties and subsequently demonstrated the ability to prevent colectomy (52,103).
More recently, a biosimilar to infliximab (CT-P13 SC) has demonstrated efficacy for (SC) dosing in maintenance phase of moderate-to-severe UC. Five hundred forty-eight patients with moderately to severely active UC and inadequate response or intolerance to conventional therapy received open-label CT-P13 5 mg/kg IV at weeks 0, 2, and 6. At week 10, clinical responders were randomized 2:1 to CT-P13 120 mg or placebo every 2 weeks–54 weeks. Clinical remission rates at week 54 were statistically significantly higher with CT-P13 SC compared with placebo (43.2% vs 20.8%; P 186). The SC dosing schedule has higher serum concentrations of drug than are seen at trough with standard IV dosing. The clinical benefit of these pharmacokinetics remain to be fully explored (187).
In the Ulcerative Colitis Long-Term Remission and maintenance with Adalimumab-2 trial, 494 patients with moderately to severely active UC were randomized to receive adalimumab 160 mg SC at week 0, 80 mg SC at week 2 followed by 40 mg every other week starting at week 4, or placebo (179). The primary endpoint, induction of remission at week 8, was reported based on anti-TNF exposure. The overall rates of clinical remission at week 8 were 16.5% for those receiving adalimumab and 9.3% among those receiving placebo (P = 0.019); at week 52, those receiving adalimumab were in remission 17.3% of the time compared with those receiving placebo at 8.5% (P = 0.004). The anti–TNF-naive patients’ rates of remission at week 8 were 21.3% for those receiving adalimumab and 11% for placebo (P = 0.017); at week 52, 22% of TNF-α–naive adalimumab patients were in remission compared with 12.4% of TNF-α patients receiving placebo (P = 0.029). However, at week 52, although patients who previously were exposed to anti-TNF agents receiving adalimumab achieved remission at week 8 more often than those who received placebo (9.2% vs 6.9%, P = 0.559), the rate was not significantly different (179).
The PURSUIT program (Program of UC Research Studies Utilizing an Investigational Treatment) evaluated golimumab in double-blind phase 2 dose-finding and subsequent phase 3 dose-confirmation trials in 1,064 patients with UC. The phase 3 results identified rates of clinical response at week 6 in patients receiving 200/100 mg and 400/200 mg golimumab of 51.0% and 54.9%, respectively, compared with 30.3% among those receiving placebo (both, P ≤ 0.0001) (180). The subsequent phase 3 maintenance study of randomized responders to induction demonstrated week 54 clinical response in 47.0% of patients receiving 50 mg golimumab, 49.7% of patients receiving 100 mg golimumab, and 31.2% of patients receiving placebo (P = 0.010, P P = 0.004 and P = 0.002, respectively) or 50 mg golimumab (23.2% and 41.7%, respectively) (181).
Two meta-analyses compared the efficacy of infliximab with the other anti-TNF agents using a network meta-analysis methodology (183,184). Although some of the comparisons did not reach statistical significance, there was a trend of higher remission rates in the UC patients receiving infliximab compared with adalimumab or golimumab. In patients with moderately to severely active UC who were naive to anti-TNF agents and immunomodulators and who had normal thiopurine methyltransferase activity, combination therapy with infliximab 5 mg/kg (loading 0, 2 and 6 weeks) and azathioprine (2.5 mg/kg orally) was superior to monotherapy with either agent alone in inducing corticosteroid-free clinical remission at 16 weeks (188). Unlike a larger, similar study in CD (189), monotherapy with infliximab was not superior to monotherapy with azathioprine in this study of patients with UC. Observational studies have compared the efficacy of infliximab and adalimumab in biologic-naive patients with UC. In a Danish study of 1,719 adults with UC, adalimumab was associated with higher rate of all-cause and UC-related hospitalizations but not abdominal surgery (190). A second study suggested lower corticosteroid usage in infliximab-treated patients with UC compared with those using adalimumab (191). There are limited data on the role of methotrexate in combination with an anti-TNF agent in UC. Extrapolating data from patients with CD, it is possible that methotrexate may offer the same benefit in terms of reducing immunogenicity and improving drug concentrations when used in combination with an anti-TNF agent and may be the preferred immunomodulator for combination therapy in those at higher risk of adverse effects of thiopurines such as young men or those with multiple skin cancers.
The anti-integrin drug vedolizumab is an effective therapy for induction of remission of moderately to severely active UC. The mechanism of this therapy (inhibition of alpha-4 beta-7 integrins) targets the mucosal immune system of the gut, and therefore, the therapy has an excellent safety profile. In the GEMINI 1 (Vedolizumab as Induction and Maintenance Therapy for Ulcerative Colitis Trial) induction trial, 374 patients were randomized in a comparison cohort to receive vedolizumab or placebo at weeks 0 and 2, while 521 patients were enrolled in the open-label vedolizumab cohort (192). Approximately 40% of these patients had failed or were intolerant to anti-TNF agents before enrollment in this study. In the comparison cohort, 16.9% and 40.9% of patients receiving vedolizumab achieved clinical remission and mucosal healing at week 6, respectively, compared with 5.4% and 24.8% of patients receiving placebo (P = 0.001 for both comparisons). Patients in the open-label cohort achieved comparable remission rates as those receiving vedolizumab in the comparison cohort. A post hoc analysis of the GEMINI 1 study also demonstrated a greater efficacy for vedolizumab in comparison with placebo at inducing remission in patients who had previously failed treatment with anti-TNF agents (193). Three subsequent systematic reviews demonstrated the superiority of vedolizumab over placebo for induction of remission in UC (183,194,195).
Similarly, vedolizumab was effective in maintaining remission in patients with UC, compared with no treatment (183,196). One systematic review and meta-analysis encompassing 4 studies with a total of 606 patients indicated that vedolizumab was superior to placebo in the maintenance of remission, with no statistical difference in adverse events or serious adverse events between the groups (194,195). In a pivotal trial of vedolizumab as maintenance therapy, patients responding to induction were randomized at week 6 to maintenance therapy with vedolizumab (300 mg IV every 8 weeks) or to placebo. A total of 40% of patients receiving vedolizumab maintained remission at week 52 compared with 16% of patients who received placebo (192).
Subsequently, a SC formulation of vedolizumab has demonstrated superiority over placebo for maintenance of remission after IV induction. The VISIBLE (Efficacy and Safety of Subcutaneous Vedolizumab in Patients with Active Ulcerative Colitis Trial) study of moderately to severely active UC provided open-label vedolizumab 300 mg at weeks 0 and 2 and randomized responders to vedolizumab 300 mg IV every 8 weeks, vedolizumab 108 mg SC every 2 weeks, or to placebo. Clinical remission at week 52 was achieved by 46.2%, 42.6%, and 14.3% of patients in the SC vedolizumab, intravenous vedolizumab, and placebo groups, respectively (vedolizumab SC vs placebo, P 197).
Tofacitinib is an orally administered small molecule that is a nonselective inhibitor of the JAK enzymes 1, 2, and 3. The OCTAVE (Tofacitinib as Induction and Maintenance Therapy for Ulcerative Colitis Trial) 1 (n = 598) and OCTAVE 2 (n = 541) induction trials were conducted to assess the efficacy of tofacitinib 10 mg orally twice daily compared with placebo (198). Patients enrolled had moderately to severely active UC and had failed conventional therapies (half of them had previously failed anti-TNF agents). The primary endpoint was remission (total Mayo score of ≤2, no subscore >1, and rectal bleeding subscore of 0) at 8 weeks. In both trials, clinical remission at week 8 occurred in a significantly higher proportion of patients treated with tofacitinib 10 mg twice a day (BID) (18.5% and 16.6%, respectively) compared with those receiving placebo (8.2% and 3.6%, respectively). At 52 weeks, in the maintenance trial, 40.6% of patients treated with tofacitinib 10 mg BID and 34.3% of patients treated with 5 mg BID achieved remission compared with 11.1% of those treated with placebo. By design, the OCTAVE trials allowed patients who had not achieved response by week 8 to continue in open label with ongoing induction dosing (10 mg BID) for an additional 8 weeks. Such extended induction was successful in achieving disease response in 51.2% of the nonresponders. This extended induction strategy was included in the US label for this therapy. At 1 year, the delayed responders achieved 45.8% corticosteroid-free remission (198). In subgroup analyses and a subsequent prospective trial, patients with prior anti-TNF failure benefited from the higher maintenance dose of 10 mg twice daily in clinical response, remission, corticosteroid-free remission, and endoscopic improvement (199,200). Given these data and the fact that after dose reduction to 5 mg BID patients who relapse are not always recaptured by returning to the 10 mg dosing, it is prudent to consider 10 mg BID for maintenance in most patients. The rates of serious adverse events were comparable across the placebo and tofacitinib-treatment groups, but infectious complications were slightly more frequent with tofacitinib compared with placebo in both the induction and maintenance trials. In particular, herpes zoster occurred in 5.1% of patients treated with tofacitinib 10 mg BID compared with 0.5% of patients receiving placebo (198).
JAK inhibition is known to affect lipid transport, so a percentage of patients who receive tofacitinib will have measurable changes in their lipids. The total cholesterol:high-density lipoprotein and low-density lipoprotein:HDL ratios remain stable, however. Because of these lipid changes, the US FDA requested a phase 4 study in high-risk patients to explore the risk of major adverse cardiovascular events (MACE) with this therapy. The ORAL SURVEILLANCE (Cardiovascular and Cancer Risk with Tofacitinib in Rheumatoid Arthritis Trial) study was a randomized, open-label, noninferiority, post authorization, safety endpoint trial which recruited 4,362 patients with active rheumatoid arthritis despite methotrexate who had pre-existing cardiovascular disease and randomized them to anti-TNF therapy (adalimumab or etanercept) with methotrexate or to tofacitinib 10 mg BID or 5 mg BID with methotrexate (201). The coprimary endpoints were adjudicated MACE and cancers (excluding nonmelanoma skin cancer) and was designed to continue until there were sufficient adverse events to determine the safety of tofacitinib. In a median follow-up of 4.0 years, the incidences of MACE and cancer (primarily lung cancer) were higher with the combined tofacitinib (plus methotrexate) doses (3.4% and 4.2%, respectively) than with a TNF inhibitor (plus methotrexate) (2.5% and 2.9%). In addition, adjudicated venous thromboembolism and death from any cause were more frequent with tofacitinib at a dose of 10 mg BID than with a TNF inhibitor. This led to a label change for tofacitinib in the United States (only in the United States) to be positioned after failure of anti-TNF. It is notable that such adverse events have not been seen in the pivotal trials of tofacitinib and in real-world follow-up of this therapy, despite the fact that severe UC is a known risk factor for venous thromboembolism (VTE) complications (202). Nonetheless, tofacitinib is labeled to be used after anti-TNF failure and with caution in patients with risk factors for cardiovascular disease or VTE complications.
Subsequently, upadacitinib, a selective JAK-1 inhibitor with minimal impact on the other JAKs, has been shown to be effective for the induction and maintenance of remission of moderately to severely active UC. Two phase 3 randomized trials examined the efficacy of upadacitinib 45 mg daily or placebo in inducing remission in moderately to severely active UC. In the U-ACHIEVE induction study, 26% of patients treated with upadacitinib achieved clinical remission compared with 5% of patients receiving placebo (P 0.0001). In the U-ACCOMPLISH trial, 34% of patients receiving upadacitinib achieved clinical remission at 8 weeks compared with 4% of patients on placebo (P Post hoc analysis demonstrated symptom improvement noted as early as day 1 after treatment initiation (203). The U-ACHIEVE maintenance trial randomized clinical responders to upadacitinib to receiving upadacitinib 15 mg or 30 mg daily or placebo for 52 weeks. At the end of follow-up, clinical remission was achieved by a larger proportion of patients treated with upadacitinib 15 mg/d (42%) or 30 mg/d (52%) compared with placebo (12%; P P 0.0001 for both). Similar to tofacitinib, the higher dose of upadacitinib is more effective in maintenance phase in patients who are prior-TNF exposed, so most patients are treated with 30 mg/d in maintenance. Serious adverse effects were infrequent and similar across all treatment groups, and as with tofacitinib, herpes zoster occurred in 3 patients exposed to upadacitinib and none who received placebo. There were no significant MACE or VTE events with upadacitinib in these trials, but the US FDA extended the label used with tofacitinib to upadacitinib to use after inadequate response or intolerance to one or more TNF inhibitors, with caution in patients with cardiovascular risk factors, and to use the lowest effective dose in maintenance.
Filgotinib is an additional JAK-1 selective inhibitor which has demonstrated efficacy in moderately to severely active UC and is approved for use for this indication in Europe (204). However, this therapy is not available in the United States.
Ustekinumab is a fully human IgG1 monoclonal antibody directed against the p40 subunit of both IL-12 and IL-23. The UNIFI (Ustekinumab as Induction and Maintenance Therapy for Ulcerative Colitis) trial was a randomized double-blind trial of ustekinumab induction and maintenance in moderately to severely active UC. The study assigned 961 patients to receiving either a fixed (130 mg) or weight-based (∼6 mg/kg) dose of ustekinumab intravenous or placebo. Patients who responded to induction therapy were randomized to receiving ustekinumab 90 mg subcutaneously every 8 weeks or every 12 weeks or placebo. At week 8, a larger proportion of patients who received ustekinumab 6 mg/kg (15.5%) achieved clinical remission compared with placebo (5.3%, P P P P 205). Symptom improvement was noted as early as 7 days after the induction dose (206). There were no differences in serious adverse events between treatment and placebo groups, and notably, placebo-exposed patients had increased rates of disease-related adverse events. Long-term extension of this trial as well as observational studies in large populations have also demonstrated similar sustained benefit and safety to ustekinumab therapy in patients with UC.
Guselkumab, mirikizumab, and risankizumab are monoclonal antibodies that target the p19 subunit of IL-23 and are approved for the treatment of moderately to severely active UC.
Guselkumab has both an IL-23p19 subunit inhibitor and has a native Fc fraction that also binds to CD64 on monocytes, which is proposed to provide a distinct mechanism of interest (207). The QUASAR (Guselkumab in Patients with Moderately-to-Severely Active Ulcerative Colitis Trial) phase 2b and phase 3 induction study demonstrated superiority of guselkumab over placebo (208,209). In the phase 2b dose ranging study, patients were randomized to receive guselkumab 400 mg IV, guselkumab 200 mg IV, or placebo every 4 weeks for 3 doses. At week 12, both doses of drug were superior to placebo. In the phase 3 study, guselkumab 200 mg IV at weeks 0, 4, and 8 demonstrated that a significantly greater proportion of clinical remission compared with placebo at week 12 (22.6% vs 7.9%, adjusted ∆ = 14.9%, P 209). Patients who had achieved clinical response to guselkumab induction in either of the phase 2b or phase 3 studies were randomized in the subsequent maintenance study to 200 mg SC monthly or 100 mg SC every 8 weeks or to placebo for 44 weeks. Clinical remission at maintenance week 44 was significantly greater with SC guselkumab 200 mg q4w (50.0% [95/190]; 29.5%, 20.9–38.1) and 100 mg q8w (45.2% [85/188]; 25.2%, 26.4–33.9) compared with placebo (18.9% [36/190]) (both P 210).
In the LUCENT-1 (Mirikizumab as Induction and Maintenance Therapy for Ulcerative Colitis Trial) and LUCENT-2 trials, patients with moderately to severely active UC were assigned to receive either mirikizumab 300 mg intravenously every 4 week intervals for 3 doses or placebo for the induction period. Patients who responded to mirikizumab at week 12 were randomized to maintenance treatment with mirikizumab 200 mg every 4 weeks or placebo for an additional 40 weeks. In addition to validated measures of disease activity and endoscopic endpoints, this trial also included a novel measurement of urgency using a numeric rating scale. In the induction trial, 24.2% of patients receiving mirikizumab achieved clinical remission compared with 13.3% of patients receiving placebo (P P P P 211). Mirikizumab was associated with a significant improvement in bowel urgency and quality of life during both the induction and maintenance phases (212,213).
Risankizumab was studied in a phase 2b dose ranging study of patients with moderately to severely active UC who were all advanced therapy-experienced (mostly anti-TNF). The optimal induction dose was identified to be 1,200 mg IV every 4 weeks for 3 doses, and in the subsequent phase 3 INSPIRE (Risankizumab Induction Therapy in Patients with Moderately to Severely Active Ulcerative Colitis) trial, 20.3% of patients achieved the primary endpoint of clinical remission at week 12, compared with 6.2% of patients who received placebo (P 214) In the randomized responder maintenance study COMMAND, those who received risankizumab in induction and responded were randomized to 180 mg SC, 360 mg SC, or placebo every 8 weeks. At the primary endpoint of clinical remission at week 52, both the 180 mg and 360 mg arms were statistically superior to placebo with rates of 45%, 41% and 26%, respectively. Safety was consistent with the well-described and reassuring safety profile of this class of therapy (215). Similar to other therapies in IBD, patients who are advanced therapy-naive had substantially better results than those who were advanced therapy-experienced, supporting the recommendation to treat earlier.
Several analyses have explored whether continuing 5-ASA therapy is useful in patients with UC who have required advanced therapies with biologics or JAK inhibitors. In the OCTAVE trials of tofacitinib, concurrent 5-ASA did not improve efficacy (216). This result may have been predetermined given the inclusion criteria of these trials (and all trials of advanced therapies in moderately to severely active UC) having active UC and having failed conventional therapy previously. In addition, analyses of whether there is efficacy benefit or increased risk of relapse with continuing 5-ASA or withdrawing it, respectively, have demonstrated no benefit to continuation and no harm to stopping 5-ASA in these settings, and a subsequent cost-effectiveness evaluation suggested that it is more cost effective to stop 5-ASA after advanced treatment is needed (217–219). A formal comparative effectiveness study of 5-ASA as concomitant therapy in 5-ASA- and advanced therapy-naive patients has not been performed. Similarly, continuing or adding thiopurines or methotrexate in the setting of vedolizumab, ustekinumab, guselkumab, mirikizumab, or risankizumab has not been formally assessed, but in post hoc subset analyses, there does not seem to be the benefit demonstrated in the UC SUCCESS study of infliximab with azathioprine.
There is interest in combination therapy for treatment of UC, either as induction treatment in advanced therapy-naive patients or as salvage therapy in treatment-refractory patients. The VEGA (Guselkumab plus Golimumab Combination Therapy vs. Monotherapy Trial) study was a phase 2a proof-of-concept double-blind trial that randomized 214 patients with moderately to severely active UC to either combination treatment with guselkumab (IL-23p19 inhibitor) and golimumab (anti-TNF) or either of these treatments as monotherapy. At the primary endpoint of week 12, clinical response occurred in 83% of patients receiving combination therapy compared with 61% of patients receiving golimumab monotherapy and 75% of patients receiving guselkumab monotherapy (P = 0.0032 and P = 0.2155, respectively) (220). Future studies of such novel combinations are under way (221).
A combination of curcumin and QingDai, 2 herbal compounds with previously described anti-inflammatory properties, has been studied as a treatment for active UC. In a single double-blind, randomized, placebo-controlled study of patients with SCCAI scores in the range of moderately to severely active UC and of whom many were receiving other advanced therapies, the curcumin/QingDai preparation achieved a week 8 coprimary endpoint of clinical response and endoscopic improvement or FC reduction by ≥ 50% in 43% compared with a placebo rate of 8% (P = 0.033) (222). While no major safety signals were noted in this clinical trial, other studies have associated higher doses of QingDai with the rare development of pulmonary hypertension (223). Further data are needed to inform recommendations for use of this therapy in UC.
POSITIONING CONSIDERATIONS FOR THE PATIENT WITH MODERATELY TO SEVERELY ACTIVE UC
Recommendations
Key concept statements
Summary of the evidence
A critical challenge in the management of patients with UC is choice and sequencing of therapies. Despite the importance of this question, there are limited rigorously performed studies to provide evidence-based answers. Much of the data for comparative effectiveness in naive and biologic-exposed patients with UC are post hoc from the registry clinical trials or from indirect sources such as network meta-analysis. Observational data are susceptible to bias, including confounding and selection bias. In network meta-analysis, the comparisons are anchored on placebo. In such analyses including multiple trials, there is substantial heterogeneity of these placebo groups, which in turn affects interpretation of the comparator arms. Therefore, we determined not to use indirect or post hoc data to guide our positioning recommendations. However, there are a number of basic principles that are reasonable, and observational data can provide a real-world setting to understand comparative outcomes. As mentioned in the prior section on Goals of Management, any treatment that is selected and administered to a patient with UC should be assessed at a defined time point (6–12 weeks) to confirm its efficacy and safety, and given risk of losing response to therapy over time, a disease monitoring strategy should be incorporated, with the intention of identifying secondary nonresponse (loss of response) early and to allow subsequent adjustment in treatment to prevent complications and additional morbidity. Choice of first therapy is based on the principles outlined above but may also incorporate considerations related to the presence or history of extraintestinal manifestations. Patients with concomitant inflammatory arthritis (not necessarily arthralgias) may benefit from use of anti-TNF or JAK inhibitor therapies, and patients with concomitant inflammatory skin conditions such as psoriasis and in particular those who develop inflammatory skin conditions while receiving anti-TNF therapy may benefit from IL-23 based strategies (224). Choice of second-line therapy is dictated by similar principles, taking into account the initial therapy used. Observational data do demonstrate higher levels of clinical response, improvement in arthralgias, and endoscopic remission with upadacitinib as compared with ustekinumab in a predominantly TNF exposed population (225). Similarly, upadacitinib has demonstrated reduced rates of intravenous steroids and colectomy over 12 months as compared with tofacitinib (226).
Up to one-fifth of patients receiving anti-TNF agents may not respond initially, and an additional 10%–15% may lose response every year despite an initial benefit (227,228). There are multiple factors which may contribute to primary nonresponse or secondary loss of response, including concurrent intestinal infection, overlapping functional bowel symptoms, and importantly, inadequate therapeutic drug concentrations. There are several reasons for low serum levels of drug, including increased clearance because of increased inflammatory burden, protein loss from a permeable inflamed mucosa, the development of neutralizing antidrug antibodies, or other patient-related factors such as increased body mass index or male sex (229,230). Therefore, the approach to a patient with inadequate primary response or secondary loss of response should include careful clinical evaluation, confirmation of inflammation using objective measures (endoscopy or surrogates such as CRP or FC), exclusion of enteric infections, and assessment of serum drug concentration to address the specific contributing factors and make a decision regarding treatment options, pharmacokinetic manipulation or cycling/swapping therapies or mechanisms (231,232). In patients who have inadequate response to anti-TNF, a prospective observational study in patients with IBD demonstrated the benefit of serum drug level assessment and subsequent dose adjustment (increase) (233). However, additional studies including multiple retrospective analyses have confirmed that such an approach has little yield in patients who have antidrug antibodies (231). In patients who have nonresponse or loss of response to an anti-TNF therapy, and in whom there is an adequate serum level of anti-TNF, cycling within the class to another anti-TNF therapy is not likely to be of benefit. In these situations, swapping to a different mechanism of inflammatory control may be preferred (231,232,234), although consideration can be given to the specific formulation of anti-TNF, because there may be a role specifically for infliximab if not previously used.
The strong effect of infliximab for outcomes including short-term and long-term clinical response, clinical remission and prevention of colectomy in UC has been demonstrated consistently across studies (185). The magnitude of the effect provides evidence of the effectiveness of this agent in UC. Additional observational data support the benefit of infliximab over adalimumab in UC when considering adverse events such as hospitalization or serious infection (190). The demonstrated efficacy of infliximab may be due to various factors including the weight-based dosing or intravenous delivery.
A head-to-head prospective RCT of vedolizumab compared with adalimumab in patients with moderately to severely active UC demonstrated superiority of vedolizumab at achieving clinical remission and endoscopic improvement at 52 weeks (31.3% vs 22.5%; P = 0.006; 39.7% vs 27.7%; P
A post hoc analysis demonstrated that patients who were anti–TNF-naive were more likely to respond to vedolizumab compared with those who had received anti-TNF therapy previously (193). These data further support a strategy of considering vedolizumab early in the treatment algorithm for patients with moderately to severely active UC.
There is great interest in understanding whether the IL23p19 inhibitors (guselkumab, mirikizumab, and risankizumab) are more effective than the IL12/23p40 inhibitor (ustekinumab). There are prospective comparative trials demonstrating superiority of 2 of the p19 therapies (guselkumab and risankizumab) in patients with moderately to severely active CD, but no such data exist for moderately to severely active UC. In addition, there are no data on cycling within the class of IL23p19 drugs. The low immunogenicity of these therapies suggests that cycling because of nonresponse from antidrug antibodies is unlikely to occur (as it does with anti-TNF), and other considerations to switch within class related to purported differences in mechanism of action (i.e., related to CD64) or dosing have not been studied.
The costs of advanced therapies for UC have been rising, and in response, third-party payers have instituted a variety of cost-containment strategies that are not patient-centric. These strategies include step therapy requirements, denials of recommended treatments in favor of alternative advanced therapy options, increased copayment requirements, and frequent reauthorization practices. Insurance companies have also denied coverage for needed monitoring and drug-related assessments such as FC and serum concentrations of anti-TNF therapies. These Guidelines advise that third-party payers should not come between the patient with UC and the clinician who is taking care of them. We advocate for preservation of the doctor/nurse-patient relationship and shared decision-making with the goal of improved quality of life, which should include the absence of economic hardships from expensive co-pays or delayed treatments because of denials of preauthorizations and overly complicated reauthorization processes.
MANAGEMENT OF THE HOSPITALIZED PATIENT WITH ACUTE SEVERE UC
Recommendations
Key concept statements
Summary of the evidence
ASUC is defined as the presence of 6 or more bowel movements daily accompanied by at least one systemic sign of toxicity including tachycardia, fever, anemia (hemoglobin 30 mm/hr) (63). In children, a PUCAI ≥65 is used to define ASUC (73). Patients with ASUC should be admitted to the hospital for inpatient management, IVCS therapy initiation in addition to supportive care with fluids and electrolytes. Historical cohorts show that up to one-quarter of patients with UC may develop ASUC requiring hospitalization (235,236), resulting in colectomy in up to 40% of patients (236). Even if the rate in the modern era is lower and the index hospitalization does not result in colectomy, patients requiring hospitalization represent a subgroup at high risk for subsequent adverse outcomes including need for colectomy (237). Thresholds for hospitalization vary across institutions, and patients with UC who do not meet these criteria may require hospitalization for inpatient management. In addition, while most patients with severe colitis should be admitted to the hospital, in select cases, outpatient management with close follow-up may be appropriate. The availability of new therapies and therapeutic strategies for patients with medically resistant UC in the United States has shifted care to a greater number of patients being managed as outpatients (238), so the studies of inpatients and their outcomes have shifted in many community and academic centers in the United States to the more medically resistant or truly acutely sick or progressive patients with impending risks of significant morbidity and mortality. This is distinct to other parts of the world, where emerging IBD is still associated with a rising incidence of hospitalization (239).
We recommend initial C. difficile testing in patients with ASUC. In a retrospective observational study at a tertiary referral center, it was found that in 2004–2005, more than half of the C. difficile infected patients with IBD required hospitalization, and 20% required colectomy (240). Various tests are available for the diagnosis of CDI in this setting, the most common ones being ELISA against C. difficile toxins A + B and nucleic acid amplification tests such as PCR (29). The latter tests are more sensitive, but evidence suggests that they may result in false-positive results, particularly in patients without documented diarrhea (29). Repeat stool testing is frequently not required but has been demonstrated to improve yield in some settings and should be performed on a case-by-case basis (240). In one series, up to 47% of hospitalizations for UC were associated with CDI (241). While other cohorts have reported a lower frequency, patients with UC and CDI have a 4-fold increase in mortality, longer hospital stays, and higher rates of colectomy, emergency room visits, therapy escalation, and hospitalizations up to 1 year after the index episode (24,241–243). Patients with IBD who develop CDI also frequently lack the traditional risk factors associated with C. difficile, such as previous hospitalization or antibiotic use (244). Consequently, a high index of suspicion must be maintained. More extensive disease, more severe disease, and immunosuppression (in particular corticosteroid use) may be associated with higher risk of CDI (240,244,245). Treatment of CDI in hospitalized patients with ASUC should follow the Infectious Disease Society of America Guidelines, which suggest vancomycin or fidaxomicin first-line therapy (29,246,247).
There are several goals of endoscopic evaluation in patients with ASUC, namely to establish severity of inflammation, confirm the diagnosis (in the setting of diagnostic uncertainty), and obtain biopsies to diagnose CMV colitis. As a complete colonoscopy in patients with severe inflammation may be associated with higher rates of colonic dilation and perforation, a carefully performed flexible sigmoidoscopy with minimal insufflation by an experienced operator is sufficient for most of the patients (31,32). Although there are no standardized endoscopic activity scores specific to ASUC, endoscopic findings of deep ulcerations correlate with failure of corticosteroid therapy and possibly other medical therapies and need for rescue therapy or colectomy. In a cohort of 89 patients hospitalized with ASUC, the UCEIS was higher in patients requiring rescue therapy or colectomy (median 6) compared with those who did not (median 5, P 248). In a prospective French study of 85 consecutive patients with acute severe colitis, the presence of extensive deep colonic ulcerations was associated with nonresponse to corticosteroid therapy and need for colectomy (249). A recent retrospective review of 92 patients with acute severe colitis showed that the UCEIS score correlated with both the MES (Spearman rho = 0.762; P P 250). A UCEIS score ≥7 had a higher positive predictive value of need for colectomy when compared with an MES of 3 (receiver-operator characteristic area 0.85 vs 0.65, respectively).
CMV colitis may affect up to a third of patients with acute severe colitis refractory to corticosteroid therapy (251,252). Risk factors for CMV include medically refractory disease, treatment with corticosteroids (less consistently immunomodulators and biologics), and presence of endoscopic ulceration (253). Endoscopically, CMV has a predisposition for actively inflamed tissues; biopsies from the base of the ulcer have the greatest yield. Histologic evidence of viral cytopathic effect on hematoxylin-eosin has poor sensitivity in identifying CMV disease (254). Immunohistochemistry staining, rapid viral culture methods, or PCR-based assays are the preferred modalities to diagnose CMV disease (251,252). Although there is debate about whether CMV colitis represents a true pathogenic effect or a “bystander effect,” evidence suggests a higher rate of treatment refractoriness and need for colectomy in patients with demonstrable CMV colitis. Consequently, identification of this disease should prompt treatment with antiviral therapy in the setting of refractoriness to steroids or biologic therapy. In patients who are responding to standard treatment of UC such as intravenous steroids, studies have not demonstrated that there is an added benefit to antiviral treatment. When treating CMV colitis, the most commonly studied agent is ganciclovir, administered initially intravenously and subsequently orally for a 14-day course (251,252), with a response rate around 70%. Oral therapy with valganciclovir may also be appropriate in selected patients. Given the uncertainty about the pathogenic role of CMV in this setting, colectomy should not be deferred until the completion of the full course of treatment in nonresponders. Treatment for the active colitis should not be withheld while treating the CMV.
Features suggestive of severe colitis on plain abdominal films include a thickened colonic wall, loss of haustrations, and mucosal islands (edematous mucosa surrounded by ulcerations). In one study, presence of 3 or more dilated, gas-filled small bowel loops indicated high likelihood of nonresponse to medical therapy and need for colectomy (255). In addition, plain abdominal radiographs may be useful in identifying colonic dilation (transverse colon diameter > 5.5 cm) which predicts a worse outcome. Abdominal imaging should be in conjunction with a careful physical examination eliciting abdominal tenderness, rebound, guarding, tympany, and ileus. Cross-sectional imaging with a CT scan should be restricted to patients with a suspected extraluminal complication, perforation, and in those newly diagnosed where the distinction between CD and UC may not be apparent on sigmoidoscopy.
Close monitoring of patients with acute severe colitis is essential to identify early nonresponders to IVCS therapy who may require medical or surgical rescue therapy. Day-to-day monitoring should include assessment of vital signs, physical examination to evaluate for abdominal distension or tenderness, as well as assessment of frequency of bowel movements, presence of visible blood, abdominal pain, and systemic symptoms. Several indices have been proposed to identify nonresponders to therapy. The most widely recognized is the Oxford index where more than 8 bowel movements on day 3 of IVCS treatment or 3 to 8 bowel movements along with a CRP > 45 mg/L predicted colectomy in 85% of patients meeting the above criteria (46). By contrast, the rate of colectomy in those with partial and complete response was 40% and 5%, respectively. In children, a PUCAI score greater than 45 at day 3 or greater than 70 at day 5 predicted failure of IVCS therapy and need for salvage (256). Other parameters predicting failure of steroid therapy include hypoalbuminemia and colonic dilation (integrated into the Ho index in conjunction with number of bowel movements) (257), elevation in ESR > 75 mm/hr, and body temperature >38 °C (258).
NSAIDs have been associated with IBD-related hospitalizations and disease relapses in up to a third of patients (21). Consequently, they should be avoided in ASUC. Opioids and agents with anticholinergic side effects may precipitate colonic dilation and toxicity and have been associated with poor outcomes including risk of infections and mortality and should be avoided. With the above restrictions, management of pain in patients with ASUC is challenging and should be multimodal, relying on pharmacologic and nonpharmacologic measures. A combination approach with nonpharmacologic measures (such as heating pads), acetaminophen, in conjunction with anxiolytics and sedatives may be helpful to allay pain in a significant proportion of patients. Suspicion for paradoxical hypersensitivity to aminosalicylate therapy should be entertained in patients who have recently initiated therapy with oral or topical 5-ASA agents, and such medications should be stopped at hospitalization.
All patients hospitalized with ASUC should be closely followed by a multidisciplinary team. Surgical consultation should be obtained for patients who are failing IVCS and are initiating rescue therapy. In addition to medically refractory disease, urgent surgery is indicated for patients who develop toxic megacolon (fewer than 5% of patients with acute severe colitis), perforation, or massive hemorrhage. Delayed surgery in acute severe colitis is associated with poor outcomes and must be avoided. The preferred surgical treatment of choice is a subtotal or a total colectomy with end ileostomy. Medical rescue therapy with infliximab or cyclosporine has not been shown to increase rates of postoperative complications, and necessary surgery should not be deferred based on this exposure (259).
IBD is associated with an increased risk of VTE (260–264). This risk is particularly apparent in hospitalized patients and is proportional to severity of inflammation (263). Other factors contributing to VTE risk in these patients include loss of antithrombotic proteins, use of corticosteroids, reduced mobility, and abdominal surgery (262,265). Because many patients with IBD who develop VTE do not seem to have an underlying genetic predisposition or other risk factors (264), thromboprophylaxis with low molecular weight heparin should be given to all hospitalized patients with acute colitis. Subcutaneous low molecular weight heparin seems to be safe even in patients with active bleeding from their UC and is not associated with worsening hemorrhage (266). Administration of pharmacologic prophylaxis may additionally be associated with reduced rates of VTE posthospitalization, although this benefit has not been robustly demonstrated (267,268).
Four clinical trials have examined the role of adjuvant antibiotics in hospitalized patients with ASUC. The antibiotics studied included metronidazole (269), tobramycin (270), ciprofloxacin (271), and vancomycin (272). In each of the studies, there was no difference in the proportion of patients responding to medical therapy or needing surgery. In addition, given the known association between antibiotics and risk of C. difficile infection in this population, the use of antibiotics should be restricted to those with suspected extraluminal complications or systemic signs of toxicity.
The role of complete bowel rest and parenteral nutrition has been examined in RCTs where there was no benefit over placebo (273,274). In a trial comprising 36 patients, 6/17 patients in the control group and 9/19 patients with total bowel rest and total parenteral nutrition required surgery for treatment of their colitis (P = not significant) (274). Nutritional status should be considered in the inpatient with ASUC, and enteral nutrition encouraged unless there is evidence of toxicity and need for surgery.
Systemic IVCS are the main stay of treatment of acute severe colitis. Their efficacy was first established in an open-label series, where 49 patients hospitalized with severe colitis were administered prednisolone 60 mg per day in divided doses along with topical hydrocortisone enemas. At 5 days, 73% of patients were in remission, and only 18% reported no improvement or worsening of symptoms. On long-term follow-up, 47% of patients achieving remission were able to maintain their clinical status and only 18% required subsequent surgery (275). In a systematic review of 32 studies that included 1,948 adults receiving IVCS therapy, the mean response rate was 67% (142). Just under one-third of patients (27%) underwent colectomy during the index hospitalization. Meta-regression revealed no benefit to a dose higher than 60 mg of methylprednisolone. IVCSs can be administered as a single dose, divided doses, or a continuous drip with no difference in efficacy (276). Topical corticosteroid therapy may additionally help patients with symptoms of distal involvement. Response to IVCS is usually apparent within 3–5 days of initiation and additional response after 7 days is unlikely. Thus, prolonged IVCS therapy beyond this duration without initiation of rescue therapy cannot be recommended. In the setting of suspected CDI or CMV infection, it may be necessary to continue IVCS therapy as the effect of infection may not be separable from that of the underlying colitis. The efficacy of cyclosporine in acute steroid refractory colitis was first established in a landmark controlled trial. Twenty patients with severely active UC without response to 7 days of IVCS therapy were randomized to receive cyclosporine 4 mg/kg or placebo (277). Nine of 11 patients administered cyclosporine demonstrated a clinical response at a mean of 7 days compared with none of the patients who received placebo. Similar short-term efficacy has been demonstrated at other centers (278,279). However, on long-term follow-up, up to 80% of patients may eventually require colectomy (279,280). Patients who are thiopurine-naive at the time of initiation of cyclosporine and receive thiopurine maintenance therapy have a lower risk of colectomy than patients who were either not initiated on thiopurines or had previously failed this therapy (280–282). One study demonstrated comparable clinical response and colectomy rates with 2 mg/kg of cyclosporine compared with 4 mg/kg, suggesting that the lower dose should be preferred given similar response and lower frequency of adverse events (283). Therefore, 2 mg/kg is the targeted cyclosporine dose for treatment of ASUC, with additional studies describing drug levels in the range of 200–400 for efficacy (284,285). Although cyclosporine has similar efficacy to IVCSs (286), its use should be restricted to those failing IVCS therapy except in patients who have contraindications or intolerance to corticosteroids.
The efficacy of infliximab in the treatment of patients with ASUC has been demonstrated in small clinical trials and several observational case series. In one pilot study, 4 of 8 patients who received infliximab had clinical response by 2 weeks compared with none of the patients administered placebo (287). Infliximab was also associated with biochemical response with improvement in circulating inflammatory markers. In a pivotal RCT, 45 patients not responding to 4 days of corticosteroid therapy were randomized to a single infusion of infliximab 5 mg/kg or placebo. Among 24 patients who received infliximab, only 7 patients required a colectomy by 3 months compared with 14/21 patients receiving placebo (P = 0.017) (288). Long-term follow-up of this trial revealed continued benefit at 3 years (289). Prospective observational series confirmed the short-term efficacy of infliximab therapy in acute severe colitis (290,291). In a long-term follow-up study of 211 patients from Sweden, the colectomy-free survival rates after infliximab rescue therapy at 3, 12, 36, and 60 months were 71%, 64%, 59%, and 53%, respectively, with over half the patients achieving steroid free remission by 12 months (292). There has been growing interest in the optimization of infliximab dosing in acute severe colitis recognizing that fecal drug loss may result in subtherapeutic serum and tissue concentrations resulting in suboptimal response rate (293). A retrospective study of 50 patients receiving accelerated infliximab induction, defined as 3 induction doses within a median period of 24 days, demonstrated a lower rate of colectomy with the accelerated regimen (7%) compared with standard dosing (40%); however, the rates of colectomy at 3 months were similar between the 2 groups, suggesting that the short-term benefit may not translate into improved long-term outcomes (294). A recent open-label RCT of intensified (10 mg/kg) vs standard (5 mg/kg) first dose of infliximab for ASUC in 138 patients demonstrated no difference in clinical response at day 7 or 14, clinical remission or colectomy at month 3. However, in patients with low serum albumin (295).
Tacrolimus is a calcineurin inhibitor that has been examined in the treatment of steroid-refractory UC in both children and adults. In a double-blind placebo-controlled trial of 62 patients, the clinical response rate at week 2 was 50% with tacrolimus compared with 13% with placebo (P = 0.003) (178). Rates of mucosal healing were also superior with tacrolimus compared with placebo (44% vs 13%), and side effects were few. The optimal target serum trough levels for tacrolimus seem to be 10–15 ng/mL (177), and efficacy seems to be similar in children (296). However, there are limited data on long-term outcomes and colectomy rates (296).
In a RCT comparing cyclosporine with infliximab in patients with acute severe UC not responding to IVCS (CySIF), 115 patients across 27 institutions were randomized to receive cyclosporine (2 mg/kg for 1 week, followed by oral cyclosporine) or infliximab (5 mg/kg at weeks 0, 2, and 6) (297). Responders in both groups received treatment with azathioprine from day 7 and were followed through 98 days. At the end of the follow-up, treatment failure defined as absence of day 7 clinical response, relapse between day 7 and day 98 or absence of steroid free remission at day 98 were similar with cyclosporine (60%) and infliximab (54%). The median change in the Lichtiger score was greater at days 3 and 4 with infliximab compared with cyclosporine, but the median time to response was similar between both groups (5 days with cyclosporine and 4 days with infliximab) (298). There was no difference in the rates of mucosal healing or need for colectomy. In long-term follow up from this trial, there was no difference in colectomy-free survival based on the treatment arm. Infliximab patients were maintained with infliximab, and the cyclosporine patients had various maintenance strategies that did not include cyclosporine. Colectomy-free survival rates after 1 and 5 years of follow-up were, respectively, 70.9% (59.2%–82.6%) and 61.5% (48.7%–74.2%) in patients who received cyclosporine and 69.1% (56.9%–81.3%) and 65.1% (52.4%–77.8%) in those who received infliximab (P = 0.97) (299). A second clinical trial, CONSTRUCT (Comparison of Infliximab and Cyclosporine in Steroid Resistant Ulcerative Colitis) additionally compared differences in quality of life and health care costs between the 2 treatments. There were no differences between the 2 groups (each group consisting of 135 patients allocated to either treatment) regarding quality-adjusted survival, frequency of colectomy, time to colectomy, or adverse events (including mortality) (300). Another multicenter study, using data from the ENEIDA (Nationwide registry on genetics and environmental determinants of Inflammatory Bowel Disease by GETECCU) registry, with a total of 740 patients treated with either cyclosporine, infliximab, or sequential rescue therapy, showed a similar efficacy between the 2 treatments, including similar colectomy and mortality rates, but highlighted a lower rate of adverse effects in the cyclosporine group (301).
The choice between cyclosporine and infliximab should be made based on provider experience with each drug. Infliximab is commonly used in the outpatient management of both CD and UC, and consequently, there is greater provider familiarity with dosing and monitoring for adverse events. By contrast, because cyclosporine is used less frequently and only at select centers, its use in steroid-refractory colitis should be restricted to providers who are familiar with dosing, monitoring trough concentrations, and managing adverse effects. Because the rates of treatment failure and colectomy are significant higher in patients receiving cyclosporine who have previously failed immunomodulator therapy, infliximab may be a preferred agent in such patients. Post hoc stratified analysis of the CySIF trial additionally revealed treatment effects favoring infliximab in patients with albumin 297). In addition, patients with lower serum cholesterol or magnesium are at greater risk of neurological adverse events from cyclosporine therapy and should be considered for treatment with infliximab.
There is considerable interest in the use of cyclosporine or infliximab as salvage therapy after failure of either agent. However, data supporting long-term efficacy are scarce. In a retrospective review of patients who either received infliximab after failing cyclosporine (n = 10) or cyclosporine after failing infliximab (n = 9), the rates of remission ranged from 30% to 40% in both groups. However, severe adverse outcomes were noted in 16% including one death from sepsis, one case each of herpetic esophagitis and acute pancreatitis with bacteremia. Other observational series similarly suggest that 60% of patients require colectomy by 12 months with either cyclosporine or infliximab salvage (302,303). However, the rate of severe adverse outcomes, including infectious complications, seem to be high. This suggests that the select patients who are receiving salvage therapy should be closely monitored for such outcomes.
Patients with ASUC who have previously failed infliximab or other anti-TNF biologic therapy are a growing subgroup. Previously, if such patients had also failed immunomodulator therapy, they were not considered candidates for calcineurin therapy induction in the absence of an effective maintenance agent. Numerous retrospective and one open-label prospective study demonstrate that vedolizumab may serve as a maintenance therapy for such patients when combined with a calcineurin agent (cyclosporine or tacrolimus) for a more rapid induction of remission. In a University of Chicago retrospective study of 71 patients treated with cyclosporine or tacrolimus for induction of remission of in steroid-refractory severe UC and subsequently who received vedolizumab bridge therapy and maintenance, colectomies occurred in 33% at 1 year and 45% at 2 years, with no serious adverse events reported (304). A similar approach was described by the multicenter French and Belgium GETAID (Groupe d’Étude Thérapeutique des Affections Inflammatoires du Tube Digestif) cohort in 39 patients with UC, 36 of whom had been previously treated with one or more anti-TNF therapies. This experience described a 28% colectomy rate at 12 months; 3 patients developed mild and transient renal failure, and 1 patient developed a Campylobacter jejuni colitis while treated with vedolizumab and tacrolimus (305). A subsequent single-center open-label prospective study in Serbia enrolled 17 patients, 15 of whom responded to cyclosporine induction and received vedolizumab. At 52-week follow-up, 18% underwent colectomy and there were no serious adverse events reported (306).
There are no data on the use of adalimumab, golimumab, or vedolizumab because induction rescue therapy in ASUC and their use cannot currently be recommended in this setting. There are emerging data of JAK inhibitors for the treatment of patients with ASUC. The pharmacodynamics of these small molecules offer the potential to avoid the challenges of protein loss believed to contribute to the primary and secondary nonresponse of infliximab in this clinical setting. A retrospective case-control study of 40 patients at the University of Michigan treated with tofacitinib 10 mg BID or off-label dosing of TID (chosen by the provider and patient comfort) matched by sex and date of admission to 113 patients treated with standard options (mostly infliximab, 2 patients received cyclosporine). In this study, 6 patients (15.0%) in the tofacitinib group and 23 patients (20.4%) in the control group underwent colectomy within 90 days (tofacitinib was protective against colectomy by 90 days compared with controls [hazard ratio {HR}, 0.28, 95% CI, 0.10–0.81; P = 0.018]). However, when stratifying according to treatment dose, 10 mg TID (HR, 0.11; 95% CI, 0.02–0.56; P = 0.008) was protective, whereas 10 mg BID was not significantly protective (HR, 0.66; 95% CI, 0.21–2.09; P = 0.5) (307). The TACOS (Tofacitinib in Acute Severe Ulcerative Colitis Trial) study was a large single-center, double-blind, randomized, placebo-controlled trial of patients with ASUC in India who received tofacitinib 10 mg TID (off-label dosing) or placebo for 7 days while continuing IV steroids. Of 104 patients included, only 5 had previously received anti-TNF therapy. The day 7 response (primary endpoint) was 83% in those receiving tofacitinib compared with 59% for those receiving placebo (P = 0.007). The rates of medical rescue therapy with infliximab or colectomy were lower in the patients receiving tofacitinib at days 7, 30, and 90 (cumulative probability at day 90 was 0.13) in patients who received tofacitinib vs 0.38 in patients receiving placebo (log-rank P = 0.003). Reported adverse events were mild; 1 patient had a dural venous sinus thrombosis (308). In a subsequent uncontrolled retrospective multicenter US experience of 25 patients with ASUC, 18 patients received 30 mg upadacitinib BID and 7 patients received 45 mg of upadacitinib daily. In this experience, 6 of 25 patients underwent colectomy. One patient experienced a postcolectomy (post upadacitinib) intra-abdominal venous thromboembolic event (309).
While the emerging data on tofacitinib and upadacitinib for ASUC are promising and may have benefit in selective patients. However, the fact that these studies are either uncontrolled, in populations without prior anti-TNF exposure, or using off-label dosing of the medications limit the recommendation of JAK inhibitor therapy as a standard option for all patients with ASUC at this time. Clinicians are cautioned against using higher doses of the JAK inhibitors in combination with corticosteroids or as rescue therapy immediately after infliximab because of concerns about overimmune suppression and risks of opportunistic infections (310). We provide an algorithm for management of ASUC in Figure 3.
Algorithm for the management of hospitalized patients with acute severe UC. 5-ASA, 5-aminosalicylic acid; ASUC, acute severe ulcerative colitis; BID, twice daily; CDI, Clostridioides difficile infection; CMV, cytomegalovirus; CRP, C-reactive protein; IV, intravenous; QID, 4 times a day; UC, ulcerative colitis.
Indications for colectomy in UC include (i) ASUC or (ii) chronic refractory UC not responding to traditional medical therapy or (iii) development of dysplasia and/or carcinoma in chronic UC. We focus on the first 2 indications for the purpose of this guideline. The absolute indications for surgery in ASUC include toxic megacolon, perforation, uncontrolled severe hematochezia, or multiorgan dysfunction (311). Colectomy should also be considered in any patient who fails to progress after 3–5 days of corticosteroids. Delays in surgery can be associated with an increased risk of postoperative complications (312). Delayed surgery for ASUC is associated with increased risk of postoperative complications (313). The definition of chronic refractory UC can include either (i) individuals who are refractory to induction of remission with biologics, corticosteroids, or small molecule therapies or individuals who are corticosteroid-dependent. Patients who meet these criteria should be considered for surgery and offered early referral and consultation with a surgeon.
A systematic review demonstrated that early complications of colectomy (≤30 days postoperatively) occurred in 9%–65% of patients with UC; while late complications (>30 days postoperatively) occurred in 17%–55% of patients. Overall postoperative mortality associated with colectomy for UC was 1.0% (314). Of the various therapies for UC, prolonged corticosteroids in particular are associated with an increased risk of postoperative infectious complications in observational studies after surgery (315). In a meta-analysis of observational studies of anti-TNF use before surgery in IBD, the pooled prevalence of any postoperative complication in UC was 35%. Preoperative anti-TNF was associated with increased risk of postoperative infectious complications in CD, but not in UC (316). In a single-center retrospective cohort, a detectable level of anti-TNF (as compared with no level) was not associated with increased surgical complications in patients with UC (317). A retrospective series describing perioperative use of vedolizumab and postoperative infectious complications in patients with UC undergoing colectomy demonstrated no increased risk, although overall numbers were small (318). There are no current data on small molecules and subsequent colectomy in patients with UC.
Another factor heavily influencing surgical outcomes is nutritional status/malnourishment. Optimization of nutritional status should be considered in the period before colectomy if possible. Poor nutritional status is associated with increased in-hospital mortality, increased length of stay and costs, and increased infection rates. Definitions of malnourishment include weight loss >10%–15% in the prior 6 months, body mass index 2, and serum albumin 319,320). Enteral and/or parenteral options should be considered in malnourished patients with UC based on individual clinical scenarios.
Restorative proctocolectomy with ileal pouch anal anastomosis is currently the surgical procedure of choice for the management of refractory UC. Construction of the pouch is not performed in the first stage of the procedure for the refractory patient on medical therapies such as corticosteroids. This staged approach minimizes complications and initial operation time. Delaying the reconstruction allows for improvement in nutritional status and the ability to minimize the potential for infectious complications of UC therapies. Therefore, a multiple staged approach should be considered in patients in UC undergoing colectomy for ASUC or chronic refractory UC not responding to medical therapy.
FUTURE DIRECTIONS
While this guideline provides an evidence basis for current day management of UC, there are many arenas in which future work is needed. This guideline did not address the arenas of endoscopic surveillance or management of UC during pregnancy. These topics will be addressed in future guidelines and a pregnancy consensus statement by the ACG.
There is great interest in the application of IUS to the assessment of UC, and further research is needed to define the timing of this tool, as well as understanding the role for transmural assessment in prognosticating treatment response and outcomes. Furthermore, while it is appreciated that there are now available a large number of treatment options for UC, there remains a great need for head-to-head RCTs to clarify sequencing and positioning of these therapies, as well as understanding the role and optimal timing for elective proctocolectomy. We need such direct evidence to inform comparative effectiveness. Further data on predictive biomarkers are needed to personalize therapy selection, from both an efficacy and safety perspective, for individual patients with UC. In addition, there remains a large unmet need to break through the existing therapeutic ceiling in UC. One component to this will be novel mechanisms of action while a second will be a better understanding of combinations of therapies to improve efficacy without sacrificing safety, with an ever-present eye on costs and access issues. We also need to better understand whether earlier advanced treatment in UC improves long-term outcomes, given recent data in CD to this effect (321).
Finally, there remains a great need for further discovery and study on causality of UC, medical cures, and prevention strategies for at-risk individuals. Overall, while our evidence base has grown immensely since the last guideline, there is much to continue to learn and improve outcomes for our patients with UC. For future IBD guideline updates, the ACG has initiated a rapid update protocol, with updated guidance released annually to provide timely and practical guidance on the ever-changing field.
CONCLUSIONS
UC is an idiopathic chronic inflammatory condition of the rectum and colon which presents with variable degrees of clinical activity and severity and is associated with significant morbidity. The appropriate and updated management of patients with UC involves successful induction of both clinical and endoscopic remission followed by use of a corticosteroid-free maintenance strategy and ongoing monitoring for disease and drug-related complications. Choice of therapy for UC is based on activity, severity, extent of inflammation and prognostic factors, and may include oral, topical (rectal), or systemic therapies, as well as surgery. Patients with moderate-to-severe disease should be treated early in their disease course with therapies that have been shown to be effective in this type of disease and should not linger with suboptimal therapy or recurrent courses of corticosteroids. In general, the induction therapy selected directs the choice of maintenance therapy. Positioning considerations for UC include a single head-to-head trial in moderately to severely active UC, separation of induction and maintenance strategies for some patients with ASUC, and considerations of comorbid or coexisting extraintestinal manifestations. We advocate for preservation of the clinician-patient shared decision-making approach to therapy selection and exclusion of limitations imposed by third-party payers.
CONFLICTS OF INTEREST
Guarantor of the article: David T. Rubin, MD, FACG.
Specific author contributions: D.T.R., M.D.L.: planning the paper. D.T.R., A.N.A., C.A.S., E.L.B., M.D.L.: collecting and interpreting data. D.T.R.: drafting the manuscript. D.T.R., A.N.A., C.A.S., E.L.B., M.D.L.: critical revisions. M.D.L., E.L.B.: performed GRADE analysis. D.T.R., A.N.A., C.A.S., E.L.B., M.D.L.: approved the final draft submitted.
Financial support: None to report.
Potential competing interests: D.T.R.: consultant/advisory board–Abbvie, Abivax SA, Altrubio, Avalo Therapeutics, Bausch Health, Bristol-Myers Squibb, Buhlmann Diagnostics Corp, Celltrion, ClostraBio, Connect BioPharma, Douglas Pharmaceuticals, Eli Lilly & Co., Foresee, Genentech (Roche) Inc., Image Analysis Group, InDex Pharmaceuticals, Iterative Health, Janssen Pharmaceuticals, Odyssey Therapeutics, Pfizer, Sanofi, Takeda Pharmaceuticals, Throne, Vedanta, and research support from Takeda. A.N.A.: research support: National Institutes of Health, Helmsley Charitable Trust, and Chleck Family Foundation. Consultant to Geneoscopy. C.A.S.: Abbvie, BMS, Boomerang, Buhlmann, Celltrion, Johnson and Johnson, Lilly, Napo Pharmaceuticals, Path Healthcare, Pfizer, Prometheus Biosciences, Prometheus Labs, Sandoz, Sanofi, Takeda, Trellus Health. Speaker for CME activities: Abbvie, Johnson & Johnson, Pfizer, Takeda. Grant support: Crohn’s and Colitis Foundation, Abbvie, BMS, Celltrion, Johnson & Johnson, Lilly, Pfizer. Intellectual property\Equity interest: Dr. Corey Siegel and Dr. Lori Siegel are co-founders of MiTest Health, LLC (software company). Technology developed by MiTest Health, LLC has been licensed to Takeda. C.A.S. has an equity interest in Trellus Health and Path Healtcare. E.L.B.: consultant/advisory board: AbbVie, Boomerang, Eli Lilly, Pfizer, Takeda, Target RWE. Research support: National Institutes of Health, Eli Lilly, Helmsley Charitable Trust. M.D.L.: consultant/advisory board: AbbVie, Takeda, Pfizer, Lilly, Bristol Myers Squibb, Target RWE, Intercept, Janssen, Prometheus. Research support: Takeda, Pfizer, Lilly.
REFERENCES







