INTRODUCTION
The purpose of this article is to update the previous American College of Gastroenterology preventive care recommendations for adult patients with inflammatory bowel disease (IBD) (1). Health maintenance issues include review and update of vaccination status for vaccine-preventable diseases and screening for cervical cancer, melanoma and non-melanoma skin cancer (NMSC), and osteoporosis. The identification of depression and anxiety and the need for smoking cessation in patients with IBD will also be reviewed. To optimize preventive care among patients with IBD, coordination between the primary care provider, gastroenterology team, and other specialists is necessary. In addition, vaccine recommendations are often country or region-specific based on the prevalence infections. In this document, we focus on recommendations for North America. Colorectal dysplasia surveillance and management were not addressed in this review.
As part of this guideline preparation, a literature search was conducted using Ovid MEDLINE from 1946 to 2024, EMBASE from 1988 to 2024, and SCOPUS from 1980 to 2024. The major terms were the controlled subject headings in MeSH: IBDs, colitis, ulcerative, and Crohn’s disease. These were translated into the EMTREE controlled vocabulary as enteritis, ulcerative colitis (UC), and Crohn’s disease (CD). Words in the title for these diseases were also included. The balance of the search involved the concepts of interest, including vaccination, immunizations, specific vaccines and diseases, as well as screening, cervical cancer, melanoma, NMSC, smoking, depression, osteoporosis, etc. The results were limited to trials, meta-analyses, systematic reviews, and existing guidelines. Cohort studies and reviews were included in areas where trials were unavailable. Each author performed an updated literature search in 2024 to include recently published articles.
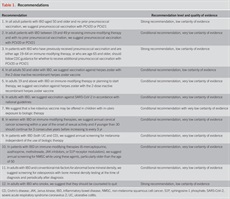
To evaluate the level of evidence and the strength of recommendations, we used the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) system (2). The level of evidence could range from “high” (implying that further research was unlikely to change the authors’ confidence in the estimate of the effect), “moderate” (further research would be likely to have an impact on the confidence in the estimate of effect), “low” (further research would be expected to have an important impact on the confidence in the estimate of the effect and would be likely to change the estimate), or “very low” (any estimate of effect is very uncertain). The strength of a recommendation was graded as “strong” when the desirable effects of an intervention clearly outweigh the undesirable effects and as “conditional” when there is uncertainty about the trade-offs. We preferentially used meta-analyses or systematic reviews when available, followed by clinical trials and retrospective cohort studies. The recommendations from this guideline are listed in Table 1. Summary statements, when listed, are designed to be descriptive in nature without associated evidence-based ratings.
Recommendations
In addition to guideline recommendations, the authors highlighted key concept statements that were not included in the GRADE assessment (Table 2). Key concepts are statements to which the GRADE process has not been applied and can include both expert opinion recommendations and definitions/epidemiological statements.

Key concepts
VACCINATIONS
Patients with IBD are at increased risk for infections as a consequence of their disease, and this risk may be amplified by certain immune-modifying therapies (3,4). Advances in the treatment of IBD have evolved with the development of tumor necrosis factor (TNF) inhibitors, interleukin (IL)-12/23 antibodies, IL-23 antibodies, anti-integrin antibodies and novel small molecules sphingosine-1 phosphate (S1P) receptor modulators, and Janus kinase (JAK) inhibitors that have enhanced the treatment response and overall quality of life of many patients with IBD. The use of these agents is associated with increased rates of clinical remission and mucosal healing; however in some cases, they are also associated with an increased risk of serious infections. Many of these serious infections, defined as those that require hospitalization, may be preventable with routine vaccination (5).
Historically, patients with IBD have been reported to have lower vaccination rates than the general population (6). One study using data from the National Health Interview Survey showed higher immunization rates among patients with IBD compared with the general population but still suboptimal rates with yearly influenza and pneumococcal vaccine uptake (48% and 75%, respectively) (7). There are many potential reasons for lower vaccine uptake among patients with IBD, such as lack of knowledge about the need for these vaccines by primary care providers, concerns about vaccine safety among patients, and lack of clarity as to which provider (primary care provider or gastroenterologist) is responsible for recommending and providing age-appropriate vaccines (8). Infectious disease clinical practice guidelines for vaccination of immunosuppressed hosts recommend that specialists share the responsibility for recommending and administering appropriate vaccines (9). Therefore, gastroenterologists should be aware of the necessary vaccines for their patients with IBD and play an active role in ensuring that their patients are up to date with the adult immunization schedule.
Simple office measures can easily be implemented to improve vaccination rates in clinical practice (10). In 1 study, vaccination rates for a high-risk IBD population were significantly improved by using a checklist as part of a quality improvement intervention (11). In other studies, making influenza and pneumococcal vaccinations available in the gastroenterologists’ office increased vaccination rates significantly (12–14). As gastroenterologists typically prescribe drugs that alter the immune system, it is incumbent that providing vaccination and health maintenance recommendations are part of the high-quality complete care offered in our practices (15). Other checklists that review vaccination and general health maintenance recommendations for patients with IBD are also available (16,17).
Principles of vaccination
Vaccines are used to stimulate the immune system to produce a response that prevents or reduces the severity of vaccine-preventable diseases. They contain antigens that result in an immune response similar to that observed after a natural infection without morbidity from the disease. Currently, several types of vaccines are available.
Key concept
LIVE ATTENUATED VACCINES
Live vaccines contain weakened or attenuated versions of viruses or bacteria that undergo limited replication upon administration and induce an immune response without causing disease. The vaccine-induced immune response to a live vaccine is similar to that observed after natural infection. The vaccine is usually effective after a single dose and the protection is of a longer duration. For example, a second dose of measles, mumps, and rubella (MMR) vaccine and varicella vaccines are used to provide an additional opportunity to induce a protective immune response that provides sustained immunity rather than as a booster after the first dose (18,19). Live vaccines are generally contraindicated in patients with IBD on immune-modifying therapy because of concerns that they may result in serious infections by attenuated viruses or bacteria. Emerging evidence suggests that certain live vaccines may be safe in some immunosuppressed patients depending on the type of immunosuppressive regimen and the live vaccine. A study of 17 children with IBD or autoimmune hepatitis receiving immunosuppressive therapy showed that varicella vaccination was safe and well tolerated (20). Furthermore, a systematic review of live vaccinations on immunosuppressive therapy in patients with immune-mediated inflammatory diseases, solid organ transplantation, or after bone marrow transplantation found that serious adverse events and infections related to varicella-zoster virus (VZV) vaccines were rare (21). While the review evaluated the safety of 8 live vaccines, more than 95% of the doses administered were of the live attenuated herpes zoster (HZ) vaccine. This is a limitation because the goal of the live HZ vaccine is to boost VZV specific cell-mediated immunity and not to induce primary protection against VZV such as the varicella vaccine and other live vaccines. In the review, 202 doses of varicella vaccine were administered to people with immune-mediated diseases on different types of immunosuppressive therapy resulting in 2 cases of varicella. Despite these limitations, disseminated infections have been reported in immunosuppressed populations after receiving a live VZV vaccine (22). Furthermore, in a retrospective study of 35 patients with IBD on immunosuppressive therapy (primarily anti-TNF agents) who inadvertently received live vaccines (MMR, zoster, or varicella), no infections or adverse events were reported within 3 months of vaccination, despite live vaccines being contraindicated in this population (23). These studies have 2 key limitations: first, significant variability in immune-modifying therapies evaluated (predominantly immunomodulators with few patients on biologics), and second, vaccination records were often incomplete, with unclear immunization histories and some patients receiving live vaccines despite prior varicella vaccination. Therefore, live vaccines (live attenuated influenza vaccine, measles-mumps-rubella, dengue, and yellow fever vaccines) are not recommended in patients with IBD on immune-modifying therapy.
Inactivated vaccines
Inactivated vaccines are not viable and cannot replicate (24). They are produced by growing pathogens in culture and by inactivating them using heat or chemicals. There are many different types of inactivated vaccines, including mRNA (COVID-19), adenoviral vector (COVID-19), recombinant (hepatitis B, human papillomavirus [HPV], and HZ subunit), toxoid (e.g., diphtheria and tetanus), and polysaccharides (e.g., pneumococcal polysaccharide). Toxoids or antigens of bacteria or viruses, along with adjuvants at times, are used to stimulate an immune response to provide protection from disease. Vaccine-induced immunity induced by inactivated vaccines has a shorter duration than that obtained from live attenuated vaccines. Therefore, multiple doses are required to provide adequate protection. The first dose typically does not induce protective immunity but primes the immune system. A protective immune response develops after the second or third dose. Because protection wanes with inactivated antigens, periodic supplemental doses, or boosters are required to maintain protection.
Key concept
Summary of evidence
Timing of vaccination and impact of immune-modifying therapies on vaccine response.
The optimal time to review vaccination status and recommend primary vaccines, boosters, or catch-up vaccines is at the time of the initial diagnosis of IBD, during the transition of care to another gastroenterology provider or during periods of remission at routine visits. Whenever possible, vaccines should be administered before immune-modifying therapy because certain agents may blunt the immune response to vaccines (25) but should not delay the initiation of appropriate therapy. Patients with IBD receiving aminosalicylates or thiopurine monotherapy mount normal vaccine responses and maintain sustained vaccine responses similar to those of healthy controls (26,27). Patients with IBD receiving anti-TNF therapy may have a blunted vaccine response to inactivated vaccines, such as influenza, hepatitis B, and pneumococcal pneumonia (28–30). Several studies have shown that those on combination anti-TNF therapy with thiopurines or methotrexate are more likely to have a blunted vaccine response (28–30). A study evaluating whether administering an influenza vaccine either concurrently with infliximab infusion or midway through the dosing interval found that the timing of infliximab had no effect on vaccine response (31). A meta-analysis of 9 studies evaluating immune response to hepatitis A and B, pneumococcus, and influenza vaccines found that immunosuppressed patients with IBD had a significantly lower vaccine-induced immune response than nonimmunosuppressed patients. Those receiving combination anti-TNF therapy had the lowest response to adult vaccines (25). Studies evaluating the immunogenicity of mRNA COVID-19 vaccine found that those on anti-TNF therapy, whether as monotherapy or in combination, was associated with lower antibody concentration. It is not known whether combination anti-TNF therapy affects the immunogenicity in those receiving a recombinant HZ vaccine.
Given that those on combination anti-TNF therapy may have a blunted vaccine immune response, the benefits of temporarily holding immune-modifying therapy have been evaluated in patients with rheumatoid arthritis. These studies showed that temporary discontinuation of methotrexate results in an increased humoral immune response to an influenza vaccine and a potential risk of disease flare (32,33). Whether holding an immunomodulator for a potentially higher antibody response is clinically beneficial is unknown. Furthermore, the results of these studies may not be generalizable to other inactivated vaccines, especially those with an adjuvant (e.g., recombinant HZ vaccine), which induces a more robust vaccine-induced immune response, or to patients taking non-TNF biologics (vedolizumab and ustekinumab), which may not affect vaccine responses.
Non–anti-TNF biologic drugs for IBD may not affect vaccine responses. Vedolizumab targets α4β7 integrin and selectively inhibits the migration of memory T cells into gastrointestinal (GI) mucosa (34,35). In a study of healthy controls, vedolizumab did not affect the vaccine response of the hepatitis B vaccine series but diminished the vaccine response to an oral cholera vaccine (35). In 2 small studies evaluating the immunogenicity of influenza vaccines, patients with IBD who received vedolizumab had a normal vaccine response (36,37). Ustekinumab is a monoclonal antibody to the p40 subunit of IL-12 and IL-23 (38). A small study of 15 patients with IBD on ustekinumab compared with 20 healthy controls failed to show that ustekinumab impacted influenza vaccine response, although the findings of this study are limited by the small sample size (39). In larger studies evaluating the immunogenicity of mRNA COVID-19 vaccines in patients with IBD, vedolizumab or ustekinumab were not associated with lower antibody responses (40–43). Whether these findings of non-TNF biologics are generalizable to other inactivated vaccines (e.g., influenza and pneumococcal vaccines) that may not produce a robust immune response is not known (42).
Oral small-molecule JAK inhibitors are approved for the treatment of UC (tofacitinib and upadacitinib) and CD (upadacitinib). Inhibition of the JAK-STAT pathway induces systemic immunosuppression and may affect the vaccine response (44). Tofacitinib was associated with a lower humoral immune response to pneumococcal vaccines but not influenza vaccines in a study of patients with rheumatoid arthritis (45). Future research is needed to determine whether newer agents for IBD, such as upadacitinib, S1P receptor modulators (ozanimod or etrasimod), and selective IL-23 inhibitors (mirikizumab, risankizumab, or guselkumab), affect the immune response to vaccines. While some agents may affect vaccine response, vaccines should be administered at the earliest opportunity, irrespective of the timing within the treatment cycle of biologic therapy, and that small molecules are not held before vaccination.
Safety of vaccinations in patients with IBD.
Several studies have demonstrated that vaccination with nonlive vaccines in patients with IBD are safe. In a systematic review and meta-analysis of 2016 patients through 2020, adverse events after vaccination were mainly local or mildly systemic and were not different from those observed in patients without IBD (46).
Key concept
Summary of evidence.
Influenza infections typically occur annually during the late fall through the early spring. Influenza virus infection results in respiratory illnesses, and most people recover without serious complications. However, influenza can result in complications in certain populations, such as older adults (65 years and older), pregnant women, persons with certain chronic medical conditions, and immunosuppressed populations, including patients with IBD (47–50). Influenza infections are associated with significant morbidity and mortality with greater than 109,000 hospitalizations and 8,000 deaths during the 2017–2018 season (51). Influenza vaccination provides protection against influenza and its potential complications (52). The ACIP recommends annual influenza vaccination for all individuals older than 6 months who do not have contraindications (52).
The ACIP has recommendations for older adults (65 years and older) who are at an increased risk of severe influenza-associated illness, hospitalization, and death compared with younger persons (48). Older adults are more likely to have a lower humoral immune response to standard-dose influenza vaccines (52). Three different types of influenza vaccines are recommended for older adults: high-dose (HD) influenza vaccine, recombinant influenza, and adjuvanted influenza vaccine (52). These vaccines are associated with higher effectiveness against influenza and are more likely to induce higher antibody concentrations than standard dose (SD) vaccines. Most studies evaluating vaccine effectiveness or immunogenicity in immunosuppressed populations have been performed using the HD influenza vaccine (53). Studies evaluating whether 1 of the 3 vaccines has superior immunogenicity compared with the others have not been performed. Thus, ACIP does not provide a preference for 1 of the 3 vaccines.
A large retrospective study found that patients with IBD were at an increased risk of influenza compared with non-IBD controls and were more likely to have complications from influenza resulting in hospitalization or pneumonia. In this study, patients on systemic corticosteroids were at an increased risk of influenza (50). Another retrospective study using national inpatient data found that influenza was the most common vaccine-preventable disease resulting in a serious infection among patients with IBD (54). Similar large retrospective studies evaluating the burden of vaccine-preventable diseases have found that influenza is a common serious infection in patients with IBD (55,56).
Similar to other inactivated vaccines, the vaccine-induced immune response to an influenza vaccine may be affected by the IBD treatment regimen. Patients receiving anti-TNF monotherapy or combination therapy (anti-TNF and immunomodulator) may have a lower immune response than those receiving immunomodulators (57–59). Multiple strategies to improve influenza vaccine response in patients receiving anti-TNF therapy have been investigated. Providing a booster dose of an influenza vaccine did not improve the humoral immune response (59,60). Furthermore, administering the influenza vaccine either concurrently with infliximab infusion or midway through the dosing interval had no effect on vaccine response (31). A randomized controlled trial (RCT) evaluating the immunogenicity of the HD vs SD influenza vaccine in patients with IBD receiving anti-TNF monotherapy found that those receiving the HD influenza vaccine had higher post immunization antibody levels (61). The overwhelming evidence shows that patients on anti-TNF therapy may have a lower influenza antibody response, but the rates of seroprotection (defined as a hemagglutination inhibition antibody titer ≥1:40, a level associated with a 50% reduction in influenza infection risk) or seroconversion (4-fold rise in antibody titers or achieving hemagglutination inhibition titer ≥1:40 from a baseline 62). This systematic review mostly focused on anti-TNF therapy and immunomodulators. As previously described in the vaccine response section, based on small studies, vedolizumab, and ustekinumab may not affect influenza vaccine response, but larger studies are needed to confirm these results. There are no data on whether JAK inhibitors, S1P receptor modulators, or IL-23 selective inhibitors affect the influenza vaccine response in patients with IBD.
Adult patients with IBD should receive annual influenza vaccination. Older adults aged 65 years and older should receive 1 of the 3 vaccines recommended by the ACIP for older adults. Furthermore, patients receiving anti-TNF monotherapy should receive a HD influenza vaccine. Ideally patients should be vaccinated in September or October, but vaccination should be offered throughout the influenza season. Coadministration of the influenza vaccine with other vaccines (pneumonia, severe acute respiratory syndrome coronavirus 2 [SARS-CoV-2], respiratory syncytial virus [RSV]) can be performed to optimize vaccine uptake (63).
Currently, a live attenuated influenza vaccine is available. It is administered intranasally and approved for individuals aged 2 through 49 years. This live vaccine is not recommended for immunocompromised patients on immune-modifying therapies because of the concern of inducing infection.
Increased risk of pneumonia and pneumococcal disease in patients with IBD
Recommendation
Summary of evidence.
Patients with IBD are at increased risk for pneumonia relative to age-matched patients without IBD (adjusted Cox proportional hazard ratio [HR] 1.54, 95% confidence interval [CI] 1.49–1.60) (64,65); this risk is apparent among both CD and UC. This risk seems increased in patients who are being treated with opioids, corticosteroids, biologic medications, thiopurines, and proton-pump inhibitors relative to patients not receiving these medications (65). Those exposed to immune-modifying therapies may be at a higher risk for hospitalization (66), and those hospitalized with pneumonia may be at an increased risk of death during hospitalization (67). In a study using the Nationwide Inpatient Sample, no increased risk of pneumococcal pneumonia was identified among individuals with IBD. However, increased risks of pneumonia attributable to influenza virus and Haemophilus influenzae were found to be increased among low-income patients with UC and all patients with IBD, respectively (55). In a cohort study of 74,156 patients with IBD and 1,482,363 non-IBD controls, the risk of invasive pneumococcal pneumonia significantly increased both before and after the diagnosis of IBD (64). In this study, there was limited impact of the use of IBD medications suggesting that the risk of invasive pneumococcal pneumonia in patients with IBD may be related to the underlying altered immune response in these patients.
There are 2 types of pneumococcal vaccines, namely pneumococcal polysaccharide vaccines (PPSVs) and pneumococcal conjugated vaccines (PCVs). PPSV vaccines currently include only the PPSV23, which is composed of partially purified pneumococcal polysaccharides from 23 serotypes prevalent at the time of the vaccine formulation in the late 1980s. PCV vaccines (currently including the PCV10 and PCV13 available for children and the PCV15, PCV20, and PCV21 for adults) include pneumococcal polysaccharides conjugated to a carrier protein to facilitate enhanced mucosal immunity.
Studies in adults with IBD have shown that immune responses to PPSV23 are impaired among patients receiving immunosuppression with thiopurines together with anti-TNF therapy (29,68). Many studies in patients with IBD and rheumatologic conditions including patients treated with anti-TNF therapy suggest that pneumococcal vaccine responses are blunted by the presence of an immunomodulator (thiopurine or methotrexate) but not monotherapy with anti-TNF (69), although a recent meta-analysis of 12 studies in IBD demonstrated lower response rates to pneumococcal vaccines among those treated with anti-TNF therapy both in combination as well as monotherapy (62). In immunocompetent adults, PCV13 seems to elicit more robust antibody responses than PPSV23 (70), although antibody persistence 1 year after vaccination was found to be similar between PPSV23 and PCV13 among patients with CD (69). Note that studies included in pneumococcal vaccine immune response assessments are primarily limited to PPSV23 and PCV13, whereas the latest guidelines (summarized below) endorse the use of PCV15, PCV20, and/or PCV21, which have not been specifically evaluated in published IBD populations to date (71). Most recently, PCV21 was approved for use in adults age 19 and older (72).
Although many published studies have examined the immunologic response rates to vaccination in IBD (62), few have evaluated the clinical impact of pneumococcal vaccination in reducing pneumococcal disease (pneumonia, sepsis, and meningitis) in the IBD population. A retrospective study using the Veteran Affairs (VA) Health Administration database in patients with IBD found that vaccination with PCV13 vaccination either alone or with PPSV23 was associated with a 5-fold decreased risk in severe pneumococcal disease, suggesting strong protective effects from PCV13 (73).
The safety of pneumococcal vaccination in the IBD population was assessed in a systematic review and meta-analysis of 13 studies that included 2,116 patients (including 4 studies evaluating PCV and/or PPSV23); authors found that most reported adverse events were local, systemic events were generally mild, and no appreciable risk of IBD disease exacerbation was identified (46).
In 2021, the US Food and Drug Administration (FDA) licensed the PCV15 and PCV20 vaccines for adults. Subsequently, recommendations for pneumococcal vaccination in adults have been adopted by the ACIP, based on evidence showing enhanced antibody responses relative to the PCV13 for the additional serotypes included in the new vaccines (71). Furthermore, recommendations for the administration of polysaccharide and conjugated pneumococcal vaccines have been simplified. These latest guidelines are relevant to adults ages 19–49 with IBD who are receiving immune-modifying therapies and to all adults with IBD age 50 and older (74).
Thus, while several strategies are outlined by the ACIP, to optimize coverage of pneumococcal pathogens and to optimize convenience, all adults with IBD regardless of age and immune suppression status who are naïve to pneumococcal vaccination should receive a single dose of PCV20 or PCV21, without the need for additional vaccines or boosters. In those patients who have previously received either PCV13 or PPSV23, see Figure 1 on the sequencing of subsequent pneumococcal vaccines. Pneumococcal vaccination can be safely administered at the same time as influenza vaccination, thus providing an opportunity to educate and target patients appropriate for pneumococcal vaccination during influenza season (63).

Key concept
Summary of evidence.
RSV infection affects the respiratory tract and can lead to significant morbidity and mortality, particularly in young infants, older adults (65 years and older), and immunosuppressed populations. It has been recognized that it poses a significant threat to older adults, carrying substantial morbidity and mortality implications (76). Despite being underreported, RSV infections in adults annually reach an estimated 1.5 million cases globally, leading to considerable morbidity and mortality, particularly in older individuals (76). RSV-related illnesses can result in hospitalization and mortality rates equal to or greater than those observed with influenza in this population (77). Serious complications such as respiratory failure and pneumonia are common among older adults infected with RSV, with mortality rates ranging from 2% to 5% (78). In addition, RSV infections can significantly affect the long-term survival of older adults, contributing to decreased 1-year survival rates compared with those infected with influenza (79). Immunocompromised populations, including solid organ transplant recipients, face heightened susceptibility to severe RSV infections and associated complications, further emphasizing the urgent need for effective preventative measures (80). In addition, a recent study found that patients with IBD are at an increased risk of serious infection because of RSV compared with non-IBD controls (81). They found that those with recent systemic corticosteroid use were at increased risk of hospitalization. Understanding and addressing the specific risks associated with RSV infection in patients with IBD are crucial for implementing effective preventive measures and optimizing patient care.
Recent advancements in RSV vaccine research have addressed this critical unmet medical need, resulting in the licensure of 3 novel vaccines that are currently recommended for adults aged 60 years and older. The FDA-approved RSVPreF3 OA vaccine (Arexvy) from GlaxoSmithKline, RSVpreF vaccine (Abrysvo) from Pfizer, and mRNA vaccine from Moderna offer promising safety and efficacy profiles, providing crucial preventive measures against RSV infections in vulnerable populations (82–84). The ACIP recommends universal vaccination for adults aged 75 and older and adults aged 50–74 years at increased risk of severe RSV because of a chronic medical condition, including cardiovascular, lung, liver and renal diseases, diabetes mellitus with end organ damage, immunocompromising conditions, obesity, neurological or neuromuscular conditions, frailty, or residence in a nursing home, should receive a single dose of RSV vaccine (85). Given the increased risk in patients with IBD, we recommend that all eligible patients with IBD receive an RSV vaccine. Furthermore, the ACIP recommends the RSV vaccine for pregnant persons at 32–36 weeks of pregnancy with a seasonal administration (September–January) to prevent RSV infections in infants (86).
Recommendations
Summary of evidence.
HZ is a painful, dermatomal cutaneous eruption that occurs most frequently among older adults and immunocompromised individuals. The disease results from reactivation of latent VZV within the dorsal root ganglia (87,88). In the general population, about 1 in 3 persons will develop zoster, or a zoster-related diagnosis, during their lifetime, with an increased incidence with age because of weakening cellular immunity. As many as 10%–18% of patients may develop debilitating postherpetic neuralgia (89).
Patients with IBD are at increased risk of developing HZ infections (90–92). The risk of HZ is higher in patients with IBD regardless of disease duration (91). In the cohort study, 7,823 CD and 11,930 patients with UC were matched on age, sex, and primary care practice to 79,563 randomly selected controls without CD or UC. In the cohort study, the incidence of zoster was higher in patients with CD and UC compared with their matched controls (UC incidence rate ratio [IRR] 1.21, 95% CI 1.05–1.40; CD IRR 1.61, 95% CI 1.35–1.92). In the nested case–control study, receipt of a prescription for corticosteroids (adjusted odds ratio [aOR] 1.5, 95% CI 1.1–2.2) or azathioprine/6-mercaptopurine (aOR 3.1, 95% CI 1.7–5.6) was both associated with zoster. In the nested case–control study, 185 patients with CD with zoster and 266 patients with UC with zoster were matched to 1,787 patients with IBD without zoster.
In another large retrospective cohort and nested case–control study using a large administrative database, 50,932 patients with CD, 56,403 patients with UC, and 1,269 with unspecified IBD, were matched to 434,416 individuals without IBD (92). The IBD cohort had an increased zoster risk compared with the non-IBD cohort (IRR 1.68, 95% CI 1.60–1.76). After adjustment for comorbidities and health care utilization, patients with IBD had a higher risk of zoster than non-IBD (HR 1.49, 95% CI 1.42–1.57). In the nested case-control multivariate-adjusted analyses, anti-TNF medications (odds ratio [OR] 1.81, 95% CI 1.48–2.21), corticosteroids (OR 1.73, 95% CI 1.51–1.99), and thiopurines (OR 1.85, 95% CI 1.61–2.13) were independently associated with zoster. The risk of zoster was the highest with a combination anti-TNF and thiopurine therapy (OR 3.29, 95% CI 2.33–4.65). In addition, HZ occurs at a younger age in patients with IBD (91). Furthermore, in a large retrospective cohort study of 4,756 matched pairs, patients with IBD were more likely to have complications of HZ than non-IBD controls (15.52% vs 12.51%; P 93).
The live attenuated HZ vaccine (Zostavax, Merck & Co) was licensed in 2006 and recommended by the ACIP in 2008 to prevent HZ and its complications in adults 60 years and older (94). It was approved by the FDA in 2011 for adults aged 50 through 59 years based on a large study of its safety and efficacy in this age group. This vaccine is no longer available in the United States but remains available in several countries including Australia and the United Kingdom.
An inactive subunit zoster vaccine containing VZV glycoprotein E and an adjuvant was effective in a study of 15,411 immunocompetent individuals aged 50 years and older reducing the risk of developing zoster by 97.2% (95). Recombinant zoster vaccine (RZV Shingrix; GlaxoSmithKline) is administered as 2 doses IM (0.5 mL each) at 0 and 2–6 months. This vaccine was first approved in 2017 for all individuals 50 years and older. In 2021, the FDA approved RZV for adults 19 years and older who are or will be at an increased risk of HZ because of immunodeficiency or immunosuppression caused by disease or therapy. In immunocompromised patients, RZV can be administered at 0 and 1–2 months as opposed to 0 and 2–6 months which is the recommendation in patients aged 50 years and older.
A retrospective cohort study of 112,220 patients with IBD 50 years and older (CD 53%, UC 47%) using the Explorys (October 2017–April 2020) dataset demonstrated that patients with IBD who did not receive RZV were at increased risk for HZ (OR 6.21, 95% CI 6.02–6.41) compared with the general population that did not receive the vaccination. Furthermore, receipt of the RZV resulted in a significantly lower rate of HZ in patients with IBD (OR 0.36, 95% CI 0.23–0.56) (96). In another retrospective VA study of 7,008 patients with IBD aged 50–60 years and 26,292 patients with IBD older than 60 years. Receipt of RZV was associated with lower risk of shingles in both the 50–60 (0.00 vs 3.93 per 1,000 person-years) and older than 60 age group (1.80 vs 4.57 per 1,000 person-years) (97). In a recent study, using the TriNetX database, 5,489 patients aged 50 years with IBD who received RZV were compared with an unvaccinated IBD control cohort followed for a mean follow-up of 901 days (98). After propensity score matching, the IBD-RZV cohort had a lower risk of HZ (aOR 0.44, 95% CI 0.32–0.62) compared with IBD control cohort. The risk of HZ was lower in patients aged 50–65 years (aOR 0.41, 95% CI 0.25–0.68) and patients older than 65 years (aOR 0.64, 95% CI 0.42–0.96). There was a lower risk of HZ in patients with UC (aOR 0.41, 95% CI 0.27–0.63) and CD (aOR 0.44, 95% CI 0.26–0.74) in the IBD-RZV cohort compared with IBD control cohort.
If the person’s history for having a chickenpox infection or varicella vaccine is unknown, it is safe for the individual to receive RZV vaccine regardless of immune suppression status. The ACIP recommends RZV for adults aged 50 years and older and immunocompromised individuals aged 19 years and older, regardless of their varicella disease or immunization status. This guidance recognizes that most adults older than age 50 have VZV exposure and that for adults 19 years and older, this approach ensures that individuals at higher risk of HZ receive protection without the need for prior varicella immunity verification. RZV, as a nonlive vaccine, poses no disease risk regardless of underlying immunity status. In a cost-effectiveness analysis, vaccination with RZV was cost-effective in all adult patients with IBD. Vaccination with RZV improved quality adjusted life years for all patients. Vaccination also reduced morbidity due to HZ by preventing these events and complications (99).
Key concept
Summary of evidence.
Primary varicella infection (chickenpox) is an infectious disease caused by VZV. Before universal vaccination, varicella was a universal childhood disease that caused a generally mild and self-limited illness but could be associated with complications in the general population with up to 4 million new infections, 11,000 hospitalizations, and 100–150 deaths per year (18,100). Varicella infections in adults are more likely to be severe and have a higher incidence of complications. Primary infections in immunosuppressed individuals are associated with significant morbidity with a high degree for disseminated disease.
Since 1995, live-attenuated varicella vaccine has been available in the United States. A single dose was originally recommended, but because breakthrough infections could occur in these individuals, a 2-dose schedule was recommended by the ACIP in 2006 (101). The 2-dose series is recommended at age 12 months and 4–6 years (18). There is a high uptake of the 2-dose varicella vaccine series among kindergarten children with greater than 92% coverage (102).
Epidemiological studies have confirmed that the varicella vaccine series is highly effective. After the introduction of the vaccine in 1996, there was an approximate 90% decline in varicella incidence. The incidence of varicella declined further in the general population after the recommendation of a 2-dose series (103). The 2-dose vaccine series decreased varicella infection rates from 800–1,600 per 100,000 in 1995 to 104). Furthermore, a meta-analysis evaluating the effectiveness of the varicella vaccine, which included 42 studies, showed 81% effectiveness for 1 dose with an increase of 92% after a second dose (105). Similarly, the vaccine has been effective in reducing the burden of varicella in the IBD population, in a large retrospective study in children with IBD, there was a decline in the number of hospitalizations for varicella since the introduction of the varicella vaccine (106). The study also found that children with IBD were at an increased risk of hospitalization because of varicella compared with non-IBD controls.
Studies in pediatric patients with IBD evaluating sustained serologic protection against varicella have reported low rates of seroprotection (107,108). This has raised concerns that pediatric patients may be susceptible to varicella infections because of the loss of immunity to VZV from their immune-modifying therapy. These studies used commercial assays to measure the VZV-specific antibody concentrations. It should be noted that the varicella vaccine induces antibody concentrations that are 10-fold lower than those in natural infections (18). Furthermore, the ACIP does not recommend using commercial assays to determine immunity to varicella if an appropriate immunization history is available. Using commercial assays to determine VZV immunity could result in a false negative as was seen in a study from the CDC where they evaluated seropositivity in healthcare workers who had received the varicella vaccine series. They found that commercial ELISA had a 34% false-negative rate compared with their more sensitive assay (109). To avoid a potential false negative result that may lead to withholding or delaying appropriate medical therapy, it would not be prudent to rely on immunization history to determine immunity to varicella in patients with IBD rather than varicella antibody testing.
The varicella vaccine should be administered to all nonimmunosuppressed patients with IBD who do not have a history of chicken pox or have not completed the vaccine series. As mentioned above, ACIP does not recommend live vaccines in populations that are considered severely immunocompromised. The vaccine series can be administered 4 weeks before the initiation of immunosuppressive therapy. If the patient is already on immunosuppressive therapy, experts from the CDC recommend waiting 3 months after discontinuation of therapy before administration (Figure 2). For patients with active IBD, treating the underlying disease with immune-modifying therapy is a priority over temporarily stopping treatment to receive the varicella vaccine series.
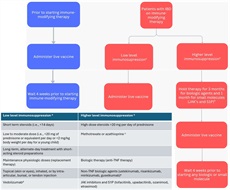
Live vaccine administration in patients with IBD. Corticosteroid therapy usually is not a contraindication to administering live-virus vaccine when administration is as listed under the low level of immunosupression column. aBased on definition from the Centers for Disease Control and Prevention Yellow Book and General Best Practices for Immunization from Center for Disease Control and Prevention. bCurrent gastroenterology guidelines have not recommended vaccination while on any immune-modifying therapy. While small studies have shown safety of live vaccines, further evidence is needed in patients with IBD. Currently, providing a live vaccine to someone on a TNF or non-TNF biologic or small molecule therapy is not recommended. For patients requiring vaccination, a potential opportunity for therapy interruption would be after surgical resection for Crohn’s disease. The decision to hold therapy requires carefully balancing infection risk (likely low even during community outbreaks) against potential consequences of treatment disruption. Notably, interrupting therapy for 3 months, which may be equivalent to episodic dosing, may result in the development of antidrug antibodies, loss of response, infusion reactions, and/or disease flares. Given these considerations, patients and their IBD providers must thoughtfully evaluate these competing risks, particularly in areas experiencing active outbreaks. cPatients on low-dose immunomodulators, methotrexate dFDA label states live vaccine may be administered concurrently with vedolizumab only if the benefits outweigh the risks. eAlthough non-TNF biologics (IL-12/23 and IL-23 inhibitors) demonstrate lower risk of serious infections compared with anti-TNF therapies, this reduced infection risk does not indicate safety for live vaccinations during treatment with these agents. IBD, inflammatory bowel disease; IL, interleukin; JAK, Janus kinase; MMR, measles, mumps, and rubella; PCV, pneumococcal conjugated vaccine; TNF, tumor necrosis factor.
Recommendation
Summary of evidence.
Patients with IBD are susceptible to acquiring COVID-19, caused by the SARS-CoV-2 virus, at rates similar to or perhaps even lower than the general population, and IBD therapies do not seem to augment this risk (110–114). Among those infected with SARS-CoV-2, IBD is not associated with worse outcomes from COVID-19 relative to those without IBD, in the absence of general risk factors known to increase the risks of COVID-19 complications such as older age, obesity, and the use of chronic corticosteroids (41,110,113).
Vaccination against COVID-19 among patients with IBD has been extensively studied in multiple observational cohorts worldwide, including mRNA vaccines and adenoviral vector vaccines (40,43,115–119). Most studies have evaluated humoral immune responses to vaccination. These studies have generally demonstrated robust responses to vaccination regardless of the IBD treatment regimen, although patients receiving anti-TNF therapies or corticosteroids at the time of vaccination may have lower postvaccination titers than those receiving other biologic agents or mesalamine alone (43,117), and postvaccination titers are lower among recipients of adenovirus vector vaccines than with mRNA vaccines (120).
Studies evaluating cellular immunity have demonstrated generally robust T-cell function, with a suggestion that both clonal T-cell expansion and functional responses may even be augmented among those on anti-TNF therapy (121–126). Epidemiologic studies have suggested that death from COVID-19 among patients with IBD was not greater than the general population and that vaccination protected against COVID-19 mortality even among those on various immune-modifying therapies (127,128). Vaccination against SARS-CoV-2 in patients with IBD is safe and well-tolerated (129,130). Postvaccination symptoms among patients with IBD tend to be less frequent than among healthy controls after both initial and booster dosing (127) and are generally mild and limited in duration (124,128,131). Furthermore, despite robust postvaccination immune responses, there is no appreciable risk of IBD disease flare after vaccination against SARS-CoV-2 with mRNA vaccines (130,132).
Key concept
Summary of evidence.
Given that patients with IBD are at an increased risk for infections, all members of their household should be up-to-date with their age-appropriate vaccine according to the pediatric or adult immunization schedule. The ACIP recommends that all household contacts and other close contacts of persons with an altered immunocompetence should receive vaccines of all age and exposures, with the exception of the smallpox vaccine (133).
They also provide the following recommendations for administering live vaccines.
- MMR, varicella, and rotavirus vaccines should be administered to contacts when indicated. They recommend the following precautions for varicella and rotavirus vaccine recipients.
- If a varicella vaccine recipient develops a rash postimmunization, they should avoid contact with a person with altered immunocompetence until the rash resolves.
- All contacts should wash their hands after changing the diaper of an infant who received rotavirus vaccine. Shedding may occur up to 1 month after the last dose.
Key concept
Summary of evidence.
All adult patients with IBD, regardless of immunosuppression status, should receive nonlive vaccines in accordance with national guidelines published by the ACIP (134), including HAV, HPV, tetanus, and pertussis.
TDAP VACCINATION IN PATIENTS WITH IBD
All adult patients with IBD should receive 1 dose of Tdap, the Td or Tdap booster every 10 years in accordance with national guidelines. Furthermore, women should receive a dose of Tdap during the 27th through 36 weeks of each pregnancy. Tetanus toxoid vaccines are also used in wound management for clean minor wounds and contaminated wounds (dirt, feces, and saliva). Those lacking evidence of having received Td or Tdap in the previous 5 years should receive a Tdap.
At least 2 studies have demonstrated that adults with IBD on anti-TNF medication may have lower antibody concentrations to pertussis (26,135). A prospective trial showed that adults with IBD on an anti-TNF medication generally responded appropriately to Tdap vaccination, although those on combined immune suppression with an anti-TNF drug and thiopurines had lower response rates (136).
HAV VACCINATION IN PATIENTS WITH IBD
Hepatitis A is transmitted through oral-fecal transmission, often through contaminated food. Vaccination against HAV is recommended by the ACIP in all children aged 12–23 months and in unvaccinated children up to the age of 18 years (134). Vaccination against HAV in adults is not universally recommended, but rather is targeted toward adults at increased risk for HAV. Adults at increased risk for HAV infection and for whom vaccination is recommended include international travelers, men who have sex with men, illicit drug users, people experiencing homelessness, and those with occupational or close contact with HAV exposure. Furthermore, those at severe risk of acquiring HAV include people with chronic liver disease or HIV. Thus, in these patients the ACIP recommends vaccination against HAV with either a single-antigen HAV or combination antigen HAV/hepatitis B inactivated vaccines (134).
There is limited evidence regarding HAV vaccination in adults and children with IBD. One study of 419 adults demonstrated an overall seroconversion rate over 97%, although this was slightly diminished among anti-TNF recipients (137). In children with IBD, HAV vaccination has been found to be safe and immunogenic (138,139).
HPV VACCINATION IN PATIENTS WITH IBD
HPV infection is associated with cervical, penile, vaginal, vulvar, oropharyngeal, and anal cancers. General guidelines recommend a 2-dose or 3-dose series in women and men between the ages of 9 and 14 and through the age of 26 if not vaccinated at younger ages. For adults aged 27–45 years, HPV vaccination should be considered through shared decision-making between patients and providers, particularly for those who may have new sexual partners and are at risk of acquiring an HPV infection. Vaccination against HPV in women with IBD seems to be suboptimal, as has been demonstrated in multiple countries (140,141). One study of 37 female adolescents with IBD showed that HPV vaccination is immunogenic and safe (142). A meta-analysis demonstrated that there was no increased risk and of IBD diagnosis after HPV vaccination with the AS04 adjuvanted HPV 16/18 vaccine (143).
MENINGOCOCCAL VACCINATION IN PATIENTS WITH IBD
Meningococcal disease is a severe illness caused by the bacterium Neisseria meningitidis. This bacterium is the leading cause of bacterial meningitis and sepsis in the United States. It can also lead to pneumonia or septic arthritis. A large claims database study evaluated the risk of meningitis among 109,859 patients with IBD compared with 296,801 non-IBD comparators. They found a low incidence of meningitis with 162 cases of meningitis among the IBD group. The incidence of meningitis was 27.6/100,000 person-years (95% CI 22.3–34.2 per 100,000 person-years) for those with CD, 20.7/100,000 person-years (95% CI 16.6–25.9 per 100,000 person-years) for those with UC and 12.7/100,000 person-years (95% CI 11.2–14.5 per 100,000 person-years) for matched comparators (144). They also found that patients with IBD were at an increased risk compared with non-IBD controls. Those treated with mesalamine therapy (OR 0.40, 95% CI 0.26–0.62) were at lower odds for meningitis and those with at least 1 comorbidity had a higher risk for meningitis (OR 2.21, 95% CI 1.76–2.77).
Three meningococcal conjugate vaccines covering serogroups A, C, W, and Y (MenACWY) and 2 meningococcal serogroup B (MenB) vaccines are licensed in the United States. The ACIP recommends the MenACWY vaccine for individuals including all adolescents aged 11–12 and a repeat dose at age 16 years. The vaccine is also recommended for individuals at increased risk such as college students living in residential housing who were not immunized routinely as adolescents, military recruits, certain travel destinations, those with asplenia, and very specific immunocompromising conditions, including those with HIV (145). As stated in the ACIP recommendations, patients with IBD with risk factors for meningitis should receive meningococcal vaccines.
Key concept
Summary of evidence.
Given the importance of hepatitis B virus (HBV) infection in patients with IBD, specific attention should be paid to assessing HBV status. Reactivation of hepatitis B has been reported in immunosuppressed patients with IBD with serious consequences (146). Testing for HBV infection (hepatitis B surface antigen [HBsAg], hepatitis B core antibody [HBcAb], and hepatitis B surface antibody [HBsAb]) and vaccination of the nonimmune patient is recommended before starting an anti-TNF medication (1,147,148). Seroprotection against HBV is determined by measuring the levels of HBsAb in the serum with concomitant negative HBsAg and HBcAb levels. There is some debate about the titer level that represents adequate protection against hepatitis B, with some groups recommending titers above 10 mIU/mL and others above 100 mIU/mL. This value is also an indicator of clinical protection against HBV infection (148). Even if the HBsAB levels wane to 149,150). Studies in immunocompetent individuals have shown that most vaccine responders to hepatitis B who have HBsAB 151–153). The ACIP recommends administering a hepatitis B challenge dose to elicit an anamnestic response and to determine the true seroprotection status. A recent study showed that adult patients with IBD immunized as children or before their IBD diagnosis have high rates of seroprotection if provided with a challenge dose to elicit an anamnestic response (154).
In a systematic review and meta-analysis of 14 studies comparing 2,375 patients with IBD to healthy controls, the pooled OR of developing seroprotection in patients with IBD was 0.13 (95% CI 0.05–0.33, P = 0.001). The effective immune response was 39.7% (95% CI 30.7–49.5, P = 0.04) (155). In healthy individuals, seroprotection after the primary 3 dose HBV vaccine series is >95% (156). By contrast, studies on HBV vaccination in patients with IBD have reported seroprotection rates of 33%–76% (157). The efficacy of different vaccination strategies against HBV has been reviewed by Marin et al. (158) In 1 study of 148 patients, 41% of those receiving single doses of Engerix-B at 0, 1, and 6 months attained seroprotection compared with 75% of patients receiving a faster, double-dose protocol (double doses of Engerix-B at 0, 1, and 2 months) (30). In 1 study of 106 patients with IBD, the use of the 2 dose adjuvanted HBV vaccine Heplisav-B resulted in the development of seroprotection in 78.3% of patients not immune to HBV (159). In a follow-up study, Karime et al reported that in patients with IBD lacking HBV immunity despite a 2-dose Heplisav-B vaccination, administration of a third dose resulted in 56.7% seroprotection (160). Similarly, a prospective study of patients with IBD demonstrated a 72% seroprotection rate after Heplisav B vaccination, particularly among adults older than age 40, previous nonresponders, and those on biologic therapy, with added benefit observed after a third vaccine dose (161). In a Markov model, Heplisav-B is more cost-effective than Engerix-B in patients with IBD receiving immunosuppressive therapy for IBD (162).
Although routine testing of titers in healthy individuals after HBV vaccination is not recommended, immunocompromised patients should have hepatitis B surface antibody levels checked 1–3 months after completion of the vaccination series to assure seroprotection was achieved (9,163–165). Prophylaxis regimens for patients at risk for HBV reactivation were recently reviewed (147,148).
Recommendation
New recommendations have emerged with respect to rotavirus vaccination in children born with in utero exposure to biologics. In a review of studies of infants exposed to biological agents (predominantly anti-TNFs) in utero and subsequently administered live-attenuated vaccines, 56 infants who received the rotavirus vaccine before the age of 6 months were described. No serious adverse events were reported among the infants (166). Similarly, a recent systematic review of the rheumatology literature evaluated the outcomes of live-attenuated vaccines in infants younger than 12 months exposed to biological agents in utero, including 46 cases of rotavirus vaccination. Only 7 mild reactions to the vaccine were reported (167). In a subsequent study, Fitzpatrick et al prospectively assessed the safety of administering the rotavirus vaccine to 168 infants exposed to biologic agents (including anti-TNFs, vedolizumab, and ustekinumab), with no reports of serious adverse events after immunization (168). In the most recent series of 57 infants born in 52 mothers with IBD receiving several different biological agents in the third trimester, there were no adverse events to 50 infants who received the rotavirus vaccine at a median age of 13 weeks (169). In a systematic review published in 2024, there was no increased risk of adverse events after rotavirus vaccine administration in over 300 biologic exposed infants (170).
OTHER HEALTH MAINTENANCE ISSUES
In addition to vaccination issues, it is important to identify subgroups of patients with IBD that have an increased risk of developing cervical cancer, NMSC, and melanoma. Additionally assessing bone health, screening for depression, and recommending smoking cessation are important measures to address when caring for patients with IBD.
Recommendation
Summary of evidence.
Cervical cancer is caused by persistent infection with certain oncogenic types of HPV. Known factors associated with an increased risk of cancer include cigarette smoking and a compromised immune system. Although vaccination against HPV remains a recommendation for women aged 9–26 years, most female patients would have been exposed to HPV by the time they are vaccinated so regular screening remains the best approach to protect women from cervical cancer. In 2019, the American Society for Colposcopy and Cervical Pathology published practice changing consensus guidelines regarding the evaluation and management of cervical dysplasia. Screening has shifted from cervical cytology that is, Pap smears to HPV testing (171). For those women who are on immune-modifying therapy, the current recommendation is based on recommendations for women with HIV infection. This includes baseline testing with cervical cytology, and if normal, then testing annually for 3 years; if normal, then every 3 years thereafter. If cytology is abnormal then HPV serotyping is performed. If abnormal cytology results are persistent, then colposcopy is recommended. For those women with IBD not on immune-modifying therapy, screening should be as in the general average risk population.
The data regarding an increased risk of cervical dysplasia and cancer from simply having a diagnosis of IBD are conflicting, but there is a consistent trend for the increased risk associated with the use of immunosuppressants. In addition, some data suggest that women with IBD and particularly those on immunosuppressants are screened even less frequently than every 3 years as recommended for healthy women (172,173). Data from the PharMetrics Patient-Centric Database from 1996 to 2005 demonstrated that while 70.4% of women with IBD (n = 9,356) received cervical testing at least once every 3 years, factors associated with reduced testing included Medicaid insurance (OR 0.28, 95% CI 0.19–0.41) and immunosuppressant medication use (OR 0.81, 95% CI 0.74–0.88) and factors in patients at the highest risk for abnormalities (172). Similarly, in a Manitoba province study, 54% of women with IBD received Pap smear screening but having CD, as well as exposure to immunosuppressant medications (azathioprine/6-mercaptopurine, methotrexate, cyclosporine, and/or infliximab/adalimumab) were independent predictors of lower use of Pap testing (173). The European Crohn’s and Colitis Organization states “given the excess risk demonstrated in various other contexts of immunosuppression, it is currently recommended that all women with IBD, particularly those receiving immunosuppressants, strictly adhere to a screening program of cervical surveillance and undergo vaccination against HPV, when appropriate” (174). An earlier meta-analysis found sufficient evidence to suggest an increased risk of cervical high-grade dysplasia and cancer in patients with IBD on immunosuppressive medications with an aOR of 1.34 (95% CI 1.23–1.46) (175). The analysis included 8 studies that were of varying size and population source but scored high on quality. Heterogeneity was found and was based on study type (case control vs cohort). The authors concluded based on the results from pooled data of more than 77,000 women, increased screening intervals, like those recommended for other chronically immunosuppressed women, are indicated. The most recent study to date is the experience of the nationwide cohort study from Denmark (176). Over 26,000 women with IBD were matched to women from the general population (n = 1,508,000). They found that women with CD were screened as often as healthy women but women with UC were screened slightly more often (IRR 1.06, 95% CI 1.04–1.08). Women with UC had an increased risk of low-grade (IRR 1.15, 95% CI 1.00–1.32) and high-grade lesions (IRR 1.12, 95% CI 1.01–1.25) compared with healthy controls. Women with CD had an increased risk of low grade (IRR 1.26, 95% CI 1.07–1.48) and high grade (IRR 1.28, 95% CI 1.13–1.45) lesions as well as well as cervical cancer (IRR 1.53, 95% CI 1.04–2.27). Interestingly, these investigators also demonstrated a 2-way association between IBD and neoplastic lesions because the IRR was higher for both conditions 1–9 years before IBD diagnosis. Also noted was an 8% increased risk for dysplasia for those women with a history of azathioprine use for either UC or CD; this effect was not seen for those on steroids or anti-TNF agents.
Women in a multicenter Dutch IBD prospective cohort from 2007 onwards were linked to cervical cytology and histology records from the Dutch nationwide cytology and pathology database, from 2000 to 2016 (177). Patients were frequency-matched 1:4 to a general population cohort. Data were available from 2,098 IBD women (77%) and 8,379 in the matched cohort with a median follow-up of 13 years. Cervical intraepithelial neoplasia (CIN) is classified by the extent of abnormal cells in the epithelium. The CIN2+ detection rate was higher in the IBD cohort than in the matched cohort (SDR 1.27, 95% CI 1.05–1.52). Women with IBD had an increased risk of CIN2+ (IRR 1.66, 95% CI 1.21–2.25) and persistent or recurrent CIN during follow-up (OR 1.89, 95% CI 1.06–3.38). Risk factors for CIN2+ in IBD women were smoking and disease location (ileocolonic [L3] or upper GI [L4]). CIN2+ risk was not associated with exposure to immunosuppressants.
A meta-analysis of articles published up to April 2021 included 7 cohort studies (including 94,144 patients with IBD and 53,661 004 healthy controls) and 4 case-control studies (including 20,267 cases and 60,034 matched controls) (178). The authors found a positive association between IBD and risk of abnormalities of uterine cervix with a random-effects model using both case control studies as well as cohort studies and reported an OR/relative risk (RR) 2.46, 95% CI 1.55–3.91, P
In a prospective, single-center German study, 99 consecutive patients with IBD seen in the GI department were sent to Gynecology, where a questionnaire was answered and gynecological examinations including a smear for cytology and HPV were taken (179). Participants of a general screening program constituted controls. There was a significant (P = 0.05) difference between the prevalence of abnormal smears in patients with (22%) and without (6%) immunosuppressive therapy, as well as to the healthy controls (5%). All immunosuppressants showed similarly high risks for abnormal smear results, including thiopurines, anti-TNF agents, and anti-integrins. Only 11/99 (11%) patients had positive high-risk HPV tests, which is comparable with the general population. The conclusions of the authors were that annual screening is advised, in particular those patients with IBD on immunosuppressive therapy.
In the most recent meta-analysis, Mann et al identified 5 population-based studies, including 74,310 patients with IBD and 2,029,087 reference patients, across 5 different countries (180). A pooled random effects model meta-analysis of these studies did not show a statistically significant increased risk for cervical cancer in IBD compared with reference populations (HR 1.24, 95% CI 0.94–1.63). However, stratified analysis by grade of lesion demonstrated an increased risk of low-grade cervical lesions (HR 1.15, 95% CI 1.04–1.28). Meta-analysis by disease subtype indicated no statistically significant increased risk in CD (HR 1.36, 95% CI 0.83–2.23) or UC (HR 0.95, 95% CI 0.72–1.25) or in patients treated with antitumor necrosis factor therapy (HR 1.19, 95% CI 0.64–2.21) or thiopurines (HR 0.96, 95% CI 0.60–1.50).
Investigators from the Netherlands reported on adult women with IBD and available cervical records in a nationwide cytopathology database for the incidence rates of CIN 2+ in patients exposed to immunomodulators and biologics (181). In 1981 women, 99 (5%) developed CIN 2+ lesions during a median follow-up of 17.2 years. CIN 2+ risk increased per year of exposure to immunomodulators (HR 1.16, 1.08–1.25). In multivariable analysis, smoking and 5-year screening frequency were also risk factors for CIN 2+ detection. The authors concluded that cumulative exposure to immunomodulators as well as prolonged screening periods increased the risk for cervical lesions and that intensified screening was warranted.
The most recent guidelines do not include any data on abnormal cervical cytology from use of JAK kinase inhibitors nor S1 P receptor modulators however because they are considered immune modulating expert opinion suggests that users of these agents follow the same recommendations as other therapies.
Recommendations
Summary of evidence.
Skin cancer is the most commonly diagnosed cancer in the United States (182,183). Basal cell and squamous cell skin carcinomas represent the most common types of skin cancer in the United States. Current estimates suggest that there are approximately 5.4 million basal and squamous cell skin cancers diagnosed annually in the United States occurring in about 3.3 million Americans because some individuals have more than one. Approximately 80% of these cancers are basal cell cancers. Squamous cell cancers occur less often accounting for about 20% of skin cancers. These forms of skin cancer infrequently lead to death or substantial morbidity (63). It is estimated that the actual death rate from basal and squamous cell skin cancer is thought to occur in approximately 2,000 people in the United States each year. This rate has been decreasing over time and most of the mortality occurs in elderly patients who may not have seen a doctor until the cancer had already grown quite large (184). Other people more likely to die of these cancers are those individuals who are immunosuppressed.
Melanoma, which constitutes 1% of skin cancer, causes more skin cancer deaths than squamous and basal cell skin cancers (185). There are currently estimates that approximately 98,000 new cases of melanoma will be diagnosed in the United States in 2023, with 8,000 associated deaths (186). Estimates of melanoma are approximately 20 times more common in white individuals than in Black individuals. Estimates in 2023 suggest that the lifetime risk for an individual to get melanoma is 1 in 38 (2.6%) for White individuals, 1 in 1,000 for Black individuals (0.1%), and 1 in 167 (0.6%) for Hispanic individuals in the general population. Survival is most dependent on early detection. Individuals who are detected early and localized disease and have their lesions excised have >99% 5-year survival whereas those individuals who have distant spread of disease have 32% 5-year survival (187).
Widespread use of biologic therapy, immunomodulators, and small molecules has led to the recognition of potential malignant complications associated with their use. Different agents used to treat patients with IBD include immunomodulators—for example, azathioprine, mercaptopurine, and methotrexate; small molecules including JAK inhibitors (tofacitinib and upadacitinib), S1P receptor modulators (ozanimod and etrasimod), anti-TNF therapy (infliximab, adalimumab, certolizumab pegol, and golimumab), anti-integrin therapy (vedolizumab), and anti-cytokine therapy (ustekinumab, mirikizumab, risankizumab, and guselkumab) has been used. An association has been established with NMSC and past or current use of thiopurines and a potential for melanoma in patients with IBD or those exposed to anti-TNF therapy. NMSC includes many different subtypes of skin cancer including angiosarcoma, basal cell carcinoma, cutaneous B-cell lymphoma, dermatofibrosarcoma protuberans, Merkel cell carcinoma, sebaceous carcinoma, and squamous cell carcinoma of the skin. The most common forms of skin cancer in patients with IBD are NMSC, basal cell carcinoma, and squamous cell carcinoma.
It is suggested that all individuals who are initiating immunosuppression therapy for the treatment of IBD should use sunscreen that is protective against ultraviolet A and B light as well use sun protective clothing. There have been no randomized trials performed in patients with IBD assessing this recommendation; however, early detection, including performing regular skin self-examinations and physician skin examinations, has been found to result in melanoma diagnoses at earlier stages, when the disease is most treatable. Thus, it is suggested that all patients with IBD should follow a program of sun protection and dermatological surveillance, which takes into account their other non–IBD-related risk factors for skin cancer development (188,189). In addition, education on skin cancer risk and rapid referral for skin abnormalities is appropriate in this patient population.
Skin cancer screening for melanoma has been demonstrated to be cost effective (185). In addition, a cost-effectiveness study highlighted that annual skin cancer screening in patients with CD is cost-effective for detection of NMSC (190). There are no RCTs to assess the effectiveness of skin cancer screening in patients with IBD. It is perceived that early detection of skin cancer can result in skin cancer diagnosis at an earlier stage. Early-stage diagnosis of skin cancer is perceived to enable the disease to be more easily treatable, especially melanoma. Because of these data, it is advocated that all patients with IBD on systemic immunosuppressive therapy should undergo a total body skin exam to screen for melanoma and NMSC annually. In addition, regular skin self-examination is advocated with patients who have IBD. Although not evidence based, this logical approach has been suggested by some individuals (191). In addition, based on the results from the CESAME study, skin surveillance strategies need to be maintained even after stopping thiopurine therapy, which differs from the recent date of the nationwide VA cohort study (192).
EPIDEMIOLOGY OF MELANOMA AND NMSC IN PATIENTS WITH IBD
Risk of melanoma in IBD
A 2014 meta-analysis (193) demonstrated a cumulative pooled crude incidence rate of melanoma in patients with IBD to be 27.5 cases/100,000 person-years (95% CI 19.9–37.0). This meta-analysis suggested that IBD was associated with a 37% increase in risk of melanoma (12 studies: RR 1.37, 95% CI 1.10–1.70) compared with the general population. In this study, the risk for melanoma was demonstrated to be increased among patients with CD (7 studies: RR 1.80, 95% CI 1.17–2.75) and UC (7 studies: RR 1.23, 95% CI 1.01–1.50). The assessment of the risk of melanoma was higher in studies performed before patients were treated with biologic therapies (studies done before 1998; 8 studies: RR 1.52, 95% CI 1.02–2.25). Based on this meta-analysis, IBD has been suggested to be associated with an increased risk of melanoma, independent of the use of a biologic.
Risk of melanoma in biologic users
In addition to concerns that IBD raises the risk of malignancy in patients with IBD, there has been a concern there may be an escalated risk of malignancy in patients using anti-TNF therapy. Based on the currently available data, there is not perceived to be a higher rate of other malignancies (i.e., nonmelanoma malignancies) in general in patients with IBD who use anti-TNF therapy. A systematic review and meta-analysis published in 2016 of 49 randomized placebo-controlled trials involving a total of 14,590 patients highlighted that there was no escalation of malignancy risk associated with biological therapy in IBD (OR 0.90, 95% CI 0.54–1.50) (194). It has also been suggested that use of an anti-TNF agent may escalate the risk of melanoma. Although this is perceived to be the case, there are inadequate data to conclusively state this. A meta-analysis demonstrated that biologic treatment was not significantly associated with melanoma in patients with IBD (pRR 1.20, 95% CI 0.60–2.40), RA (pRR 1.20, 95% CI 0.83–1.74), or psoriasis (HR 1.57, 95% CI 0.61–4.09) compared with those who received conventional systemic therapy. There was a numeric difference. but the differences were not statistically significant. Adjustment for other risk factors was absent from most studies (195).
An analysis of Danish patients found no association between anti-TNF therapy and melanoma (196), and a recent meta-analysis highlighted that there was no detectable difference in the risk of acquiring melanoma in patients with IBD who received biologic therapy compared with patients with IBD treated with nonbiologic therapies (195). Patients with IBD are likely to have the same risk factors as the general population, but in addition have treatment specific risks. There is not perceived to be an increased risk of melanoma in thiopurine exposed patients with IBD. There have been 2 meta-analyses that were performed and did not demonstrate any suggestion of an increased melanoma risk in these thiopurine-exposed patients with IBD (197,198).
Risk of NMSC with thiopurine use
There is no suggestion that IBD itself increases the risk for NMSC. Several earlier studies did suggest an independent risk for NMSC in patients with IBD, but careful evaluation demonstrates that they failed to consider the patients’ use of antimetabolite therapy. The cumulative data on NMSC risk in patients with IBD highlight that there is an increased risk with the use of immunosuppression, particularly thiopurine use. Several systematic reviews and meta-analyses have reported an increased NMSC risk in patients with IBD exposed to thiopurines (197,199,200). A large study which analyzed 13 studies among which there was a total of 149,198 patients reported that the risk of NMSC was elevated (RR 1.88, 95% CI 1.48–2.38). It is perceived that the total cumulative thiopurine dose likely influences the NMSC risk for the patient (192,201,202). When assessment of the risk for incident NMSC was determined, it was found that the IRR increased with the duration of use—1.6 in the first year and 3.6 in the fifth year (P 192,200). There have been discordant results reported on the persistent NMSC risk after thiopurine discontinuation. Several large population cohort studies suggested that the risk of NMSC resolves with discontinuation of therapy (192,201); however, several studies suggest persistent risk of a lesser magnitude (203). In addition, patients with prior basal cell carcinoma who continued thiopurine therapy were more likely to have recurrence of basal cell carcinoma (204).
Risk of NMSC with methotrexate use
A recent meta-analysis evaluated the risk of NMSC in patients with IBD treated with methotrexate (198). In this meta-analysis, there were inadequate studies in patients with IBD alone to establish firm associations; however, there were several retrospective cohort studies which have linked the patients treated for inflammatory arthritis with methotrexate to have an increased risk of skin cancer. One study reported a 4.6-fold higher incidence of basal cell carcinoma or squamous cell carcinoma (SCC) with any methotrexate use compared with no methotrexate use in patients with inflammatory arthritis (205). This risk was significantly higher for cumulative doses more than 8,000 mg (standardized incidence ratio [SIR] 4.81) compared with cumulative doses less than 5,000 mg (SIR 2.31), and a dose-response relationship was found for basal cell carcinoma only. In addition, patients receiving methotrexate for psoriasis and rheumatoid arthritis have been reported to have a slightly increased chance of developing melanoma and melanoma skin malignancies. However, this risk was not observed in methotrexate-treated patients with IBD (205–210).
Risk of cutaneous malignancy with S1P receptor modulators
There have been reports of cutaneous malignancies in patients with the use of S1P receptor modulators. There were reports of cases of basal cell carcinoma, squamous cell carcinoma, and melanoma in patients who used ozanimod. Melanoma and basal cell carcinoma was reported in clinical trials, and Kaposi’s sarcoma and Merkel cell carcinoma were reported in patients treated with S1P receptor modulators in the postmarketing setting. The actual rates and risk factors remain uncertain. Further analysis and prospective data will be required to evaluate this fully.
Risk of cutaneous malignancy with anticytokine therapy
Similarly, ustekinumab use has been linked to cutaneous malignancy. Most of the data are published on patients who used ustekinumab for psoriasis. However, it is well recognized that patients who have psoriasis are documented to be at an increased risk for skin cancer. It is somewhat difficult to discern the baseline risk of skin cancer in this patient population because many studies include prior treated patients who may have been exposed to phototherapy or prior immunotherapy which are recognized as known to increase skin cancer risk (211).
Risk of cutaneous malignancy with JAK inhibitors
Clinical trials and real-world evidence support the use of JAK inhibitors for the treatment of IBD; however, they have also been linked to adverse events, presumably an increased risk of skin cancer. Thus far however, data in patients with IBD have not suggested an escalated risk of malignancy in patients with IBD (212). There are data however from clinical trials in patients older than 50 years who had rheumatoid arthritis that has highlighted an escalated risk of malignancy compared with anti-TNF therapy, specifically NMSC, lung cancer, and lymphoma (213).
In a meta-analysis of 78 clinical trials and long-term extension studies, across all indications, JAK inhibitors was not associated with a higher incidence of malignancy compared with placebo or methotrexate (214). However, they were associated with a higher incidence of malignancy compared with TNF inhibitors. In a network meta-analysis, the incidence of all malignancies including NMSCs was not significantly different between JAK inhibitors and placebo or between JAK inhibitors and methotrexate (214). In this study, the authors combined data on multiple licensed JAK inhibitors without restriction by disease indication. This was done given the small sample size with IBD in the trials.
Risk of cutaneous malignancy with vedolizumab
Finally, the use of vedolizumab has not been linked to malignancy (215). A recent analysis of clinical trials and real-world evidence did not demonstrate a rate of malignancy that was higher than expected based on the general population.
In summary, the presence of chronic immunosuppression has been linked to an increased risk for the development of squamous cell carcinomas (SCC) and to a lesser degree also basal cell carcinomas (BCC) (216–220). Most studies regarding NMSC in patients on immunosuppression have been reported in solid organ transplant patients with substantially less published regarding the incidence of NMSC in patients with IBD.
Key concept
Summary of evidence
Pathophysiology
The etiology of IBD and disease activity after periods of remission is complex and likely involves an interaction between multiple factors. Psychological stress has been reported by both caregivers and patients to exacerbate disease, but the published literature is conflicting, in part because of the inherent difficulty in studying this area and diversity of measurement tools used. However, newer studies and animal data suggest that depression and anxiety play a role in disease course. Recent research has highlighted an important role for the enteric nervous system in mediating intestinal inflammation in the presence of chronic stress (221). It has been found that chronically elevated levels of glucocorticoids drive the generation of an inflammatory subset of enteric glia that promotes monocyte-mediated and TNF-mediated inflammation through CSF. After being triggered by glucocorticoids, some glial cells release molecules that stimulate immune cells. In turn, those immune cells release molecules that would normally be used to fight off pathogens but in this case end up promoting bowel inflammation. In addition, glucocorticoids cause transcriptional immaturity in enteric neurons, acetylcholine deficiency, and dysmotility through TGF-β. The authors of this recent research verified the connection between the psychological state, intestinal inflammation, and dysmotility in 3 cohorts of patients with IBD. Together, these findings offer a mechanistic explanation for the impact of the brain on peripheral inflammation, define the enteric nervous system as a relay between psychological stress and gut inflammation, and suggest that stress management could serve as a valuable component of IBD care.
Epidemiology
Current evidence suggests that mood disorders, in particular depression and anxiety, are more prevalent in patients with IBD than the background population. The literature is somewhat confusing as studies vary with regard to inclusion of which IBD diagnosis patients have, the state of disease activity (active vs in remission), the mood disorder studied, and the measurement tool used. A recent systematic review and meta-analysis to study the prevalence of anxiety and depression in patients with IBD included 77 studies. The authors found that anxiety symptoms were present in 32.1% of patients with IBD vs 10.4% of the background population in 58 studies, and depression was found in 25.2% with IBD vs 17.9% in non-IBD controls in 75 studies. In those studies that reported on patients with CD and UC, patients with CD were 20% more likely to report anxiety (OR 1.2, 95% CI 1.1–1.4) as well as depression compared with those with UC (OR 1.2, 95% CI 1.1–1.4). It was also reported that patients with active IBD were more than twice as likely to have anxiety (OR 2.5, 95% CI 1.5–4.1) and 3 times more likely to have depression (OR 3.1, 1.9–4.9) than those with IBD in remission (222). In a more recent population based cohort study\, Umar et al examined the incidence of mental illnesses in their IBD population from the United Kingdom (223). They found that either diagnosis was associated with a higher incidence rate of depression (IRR 1.36, 95% CI 1.31–1.42) and anxiety (IRR 1.17, 95% CI 1.11–1.24) than healthy controls.
Effect on disease course
In 2004, Mittermaier et al studied the impact of depression on relapse of IBD (224). This was a prospective longitudinal study of 60 patients with IBD (both CD and UC) in remission. Patients were evaluated every 3 months for 18 months with the Beck Depression inventory (BDI), Spielberger Anxiety inventory, IBD Quality of Life Questionnaire (IBDQ), Perceived Stress Questionnaire, and the Rating for IBD Patient Concerns. At baseline, 28% of patients had depression. A higher BDI score at baseline predicted total number of relapses after 12 and 18 months.
In another study in patients receiving infliximab therapy for their CD, the impact of depression on disease course was measured. One hundred consecutive patients underwent assessment of clinical and psychological variables at baseline and at 4 weeks after infliximab initiation. The presence of major depression at baseline predicted a lower clinical remission rate. Depression was found to be an independent determinant of active disease both at baseline and re-evaluation (HR 2.27, 1.36–3.79) (225). In a case–control study of patients with active CD, anxiety and depression were measured in quiescent vs active disease (226). Anxiety and depression scores were significantly worse in those not being treated with any immune modulating therapy, and treatment with a thiopurine to achieve remission was associated with improved psychiatric survey scores. However, a later study of 139 patients with IBD, IBS, and hepatitis C enrolled in a 1-year observational cohort prospective study of patient outcomes in relation to psychological comorbidity (227), there was no relationship between depression and anxiety and total number relapses in the IBD group specifically as compared with the IBS and hepatitis C groups. In a study of patients with IBD in several Boston area hospitals, surgery seemed to increase the risk for depression and anxiety for both UC and CD, with a risk of 16% in CD and 11% in UC within 5 years of surgery (228).
Psychological stress has also been studied in IBD populations. In a Canadian study, 101 patients with CD in remission were followed prospectively for up to a year to examine clinical, biological, and psychosocial parameters as predictors of clinical relapse (229). Monthly measurements of psychological distress and perceived stress were measured. The interaction of perceived stress and avoidance coping (a coping strategy defined as taking time away from a situation) were predictors of earlier relapse (HR 7.0, 95% CI 2.3–21.8) in the 37 patients that experienced a relapse. A cohort of 75 patients in remission with UC was followed for a year, with endoscopy and long-term perceived stress measured at baseline (230). The Hospital Anxiety and Depression Scale was also used, and acute perceived stress was measured at baseline and then after 1, 3, 6, 9, and 12 months. Short-term stress (HR 10.5, 95% CI 1.01–11.0) but not long-term stress nor depression was predictive for risk of relapse. Mawdsley et al (231) studied biochemical markers in serum and rectal mucosa in patients with UC in remission vs controls. A short-term psychological stress was applied to half of the patients with UC, and the others were controls. In patients with UC, stress increased mucosal TNF alpha release by 102% and reactive oxygen metabolites by 475% as well as reducing rectal mucosal blood flow by 22% compared with patients with UC not given stress and healthy controls.
Effect of treatment
Several different treatment options may be beneficial for people with IBD who have anxiety, depression, or difficulty coping with their diagnosis. There are both pharmacological approaches and nonpharmacological approaches which can be used alone or in combination. A randomized trial of psychotherapy and relaxation on the clinical course of CD from the German Prospective Multicenter Psychotherapy Treatment study did not show an effect at 2 years for decreasing the number of acute flares (232). However, in a follow-up study of this cohort, they found in multivariate analysis that steroid intake and depression predicted worse disease outcome and that in the high utilizers of health care, a significant drop in health care utilization was noted in those treated vs not for their depression (233).
The most recognized nonpharmacologic treatment option is cognitive behavioral therapy (CBT) which has been used primarily to treat anxiety and depression but also chronic pain. This represents a form of psychotherapy, and the focus of this therapy is to identify and challenge negative thought patterns and behaviors that can increase stress, lead to worsening mood or anxiety over GI symptoms, or interfere with managing IBD. The use of CBT was studied over a decade ago in IBD and has demonstrated variable benefit in patients (234,235). While an early pilot trial suggested a positive effect on depression scores with the use of CBT (234) later in a RCT of CBT vs standard care, investigators found that 10 weeks of CBT therapy either face to face or online did not significantly decrease depression or anxiety scores of the period of the trial (235).
Another popular nonpharmacologic approach is acceptance and commitment therapy. This has a somewhat different focus; it places emphasis on accepting what a patient cannot change (the presence of their IBD and the accompanying discomfort that may be present associated with the presence of their disease). It directs individuals to focus on their thoughts, emotions, and gut sensations. This approach attempts to improve functioning and quality of life, despite having symptoms related to IBD. Despite its popularity with the lay public, scientific evidence is scarce. In the single published study, Hou et al demonstrated in a cohort of 62 patients with IBD that with a single 1-day session anxiety scores but not depression scores, decreased significantly with a continued effect out to 3 months (236). The therapy was well accepted with 100% stating they were satisfied with the program.
In an update to their earlier systematic review and meta-analysis on the efficacy of psychological therapies, Riggott et al assessed 11 new RCTs which were then analyzed with the previous 14 (237). The studies varied in patient recruitment with regard to diagnosis and disease activity at the time of enrollment. There was a significant decrease in depression and anxiety scores after treatment, an effect that was persistent at final follow-up months later.
Pharmacologic therapy has been beneficial as treatment for patients with IBD as well. In a review of 12 studies, antidepressants were found to be effective for treating both psychological and somatic symptoms in patients with IBD (238). In a systematic review and meta-analysis of the effect of standard treatments on depression and anxiety symptoms in patients with IBD, 16 studies were evaluated (239). While they found a significant decrease in depression scores from IBD treatment alone, there was a significant amount of heterogeneity among studies, and thus, the results were inconclusive.
In another more recent systematic review and meta-analysis, Weston et al studied the effect of antidepressants on patients with IBD (240). There were a total of 11 studies included: 3 placebo controlled RCTs, 2 non RCTs, and 6 other studies with various methodologies. Only the RCTs were considered of good quality. In the pooled analysis, there was a significant effect of antidepressants on depressive symptoms. Specifically, the use of serotonin and noradrenaline reuptake inhibitors improved depressive symptoms as well as anxiety scores.
Recommendation
Summary of evidence.
Bone disease is an extraintestinal manifestation of IBD which may manifest in several fashions in patients including osteoporosis, less frequently as osteomalacia (vitamin D deficiency) or rarely hyperparathyroidism. Osteoporosis is defined by the World Health Organization (WHO) as a systemic skeletal disease characterized by low bone mass and microarchitectural deterioration of bone, with a consequent increase in bone fragility and susceptibility to fracture (241). Our current standards define osteoporosis as a bone mineral density of 2.5 standard deviations or more below the mean peak bone mass (average of young, healthy adults) as measured by dual-energy x-ray absorptiometry (DEXA); the term “established osteoporosis” includes the presence of a fragility fracture. The literal translation of the term osteoporosis means “porous bone.” Although low bone mineral density (BMD) is asymptomatic, it predisposes individuals to personal and economic burdens (242,243). Osteoporosis has a large potential impact while incurring personal and economic burdens on the patient populations afflicted with this disorder.
GENERAL RISK FACTORS
In general, the bone mass of an individual adult older than 30 years reflects the bone mass accumulated during growth minus the bone mass lost because the adult peak was attained. In the general population and in patients with IBD, the risk factors for developing low bone mass defined as either osteoporosis or osteopenia, for both men and women can be separated into 2 groups: (i) those that are measurable and (ii) those that are both measurable and modifiable. Measurable risk factors include BMD, serological markers, and urinary markers of bone formation and resorption, age, and genotype. Measurable and modifiable factors include glucocorticoid therapy, treatment with drugs that could affect bone metabolism, sex hormone and vitamin D status, exercise status, nutritional status, including dietary calcium intake, weight, smoking status, and risks for trauma/fall.
Corticosteroid use
Corticosteroids are used to treat a wide variety of diseases and medical conditions. Different studies have demonstrated that 30%–50% of patients receiving corticosteroids develop fractures (244). Even with the use of low dose corticosteroids with doses equivalent to prednisone 3–10 mg a day are associated with an escalated risk of bone fractures (245,246). Corticosteroids have multiple effects on bone, both direct and indirect, that lead to abnormal bone mineral density and increased fracture risk (247). There is published literature that suggests that the daily dose of steroid use is most able to predict the fracture potential, not the cumulative dose of steroid use (246). It has been demonstrated that doses of prednisone over 7.5 mg a day confer a fivefold higher risk of spine and hip fractures. Doses as low as 2.5 mg a day of prednisone are associated with an escalated risk (1.55-fold risk) of spinal fractures (247). After a person discontinues use of corticosteroids, the risk for sustaining a bone fracture gradually declines and goes back to prior baseline within 1–2 years (246,248).
Several other risk factors are present that independently increase the risk for an individual developing a glucocorticoid induced fracture including (i) postmenopausal women and older men who are on prednisone at doses of 20 mg or more daily, (ii) low body mass index, (iii) cigarette smoking, (iv) parental hip fracture, (v) more than 3 alcoholic drinks a day, and finally (vi) receiving intravenous pulsed steroids (249,250).
Thus, there are several very important statistics of significant clinical relevance to point out regarding corticosteroids and their effect on bone density and fracture. Use of corticosteroid therapy is associated with 30%–50% increase in the fracture risk in patients receiving long-term corticosteroid therapy in both cross-sectional and longitudinal studies (246,251). The fracture risk occurs in a dose-dependent fashion. The risk of fracture occurs even at the lowest doses, the increase was chiefly noticeable with doses greater than 7.5 mg/d of prednisone-equivalent. The occurrence of fractures was noted to occur 3–6 months after treatment initiation and decreased in frequency as early as 3 months after treatment discontinuation in large population-based studies. In addition, the use of corticosteroids therapy within the past 12 months with a treatment duration longer than 90 days was associated with a decrease in femoral neck BMD and with increases in the risk of major fractures (5.4% vs 7.7%; RR, 1.25, 95% CI 1.07–1.45; P = 0.004) and hip fractures (1.1% vs 1.8%; RR 1.61, 95% CI 1.18–2.20; P = 0.003) independent of BMD values (252).
A recent systematic review and meta-analysis was performed in 2018 that evaluated 9 different studies and compared patients with IBD to the general population (253). This study demonstrated an overall escalated risk for generalized bone fracture at 1.38 (95% CI 1.11–1.73). The rate of vertebral fractures was somewhat more elevated at 2.26 (95% CI 1.04–4.90). It was also appreciated that mean BMD and Z scores were lower at all sites measured in patients with IBD compared with controls, and there was an associated escalated fracture risk. These findings were reproduced in another meta-analysis (254).
The following lists many of the disease states and conditions associated with low bone mineral mass. Hormone excess:
- Parathyroid hormone excess
- Primary and secondary thyroxin excess, endogenous and exogenous
- Cortisol excess, endogenous and exogenous
Hormone deficiency:
- Estrogen deficiency (premenopausal state may be linked with anorexia, bulimia, athletic amenorrhea, premature menopause, prolactinoma, or hypopituitarism)
- Estrogen deficiency, postmenopausal
- Testosterone deficiency, primary and secondary testicular failure
- Vitamin D metabolite deficiency, inadequate intake, or malabsorption
Miscellaneous (not necessarily mediated by hormonal abnormalities):
- Medical conditions, including postgastrectomy states, idiopathic hypercalciuria, systemic mastocytosis, and prolonged immobilization (paraplegia and quadriplegia)
- Lifestyle factors, including cigarette smoking, excessive ingestion of caffeine, and excessive sodium intake (promotes hypercalciuria)
Medications:
- Heparin
- Warfarin
- Cyclosporine A
- Methotrexate
- Tacrolimus
Most of these conditions/states associated with low bone mass are not influenced by changes in diet or lifestyle. Each of the circumstances listed could have an impact on the skeleton at any time in an individual’s life. The effect is accentuated if it occurs in conjunction with low estrogen levels after menopause.
In addition, as with low BMD, risk factors for bony fractures may be considered modifiable or nonmodifiable, as follows:
Modifiable risk factors for abnormal BMD include current tobacco smoking, below-normal body weight (especially those with low body weight that is less than 129 pounds), deficiency of estrogen, menopause before age 45, bilateral ovariectomy, premenopausal amenorrhea for >1 year, lifelong low intake of calcium, excessive consumption of ethanol, visual impairment despite adequate correction (may increase risk of falling), repeated falls, inadequate physical activity, frailty, or poor overall physical condition.
Nonmodifiable risk factors include history of fracture as an adult, family history of fractures, especially among first-degree relatives, White, female sex, dementia, elderly, frailty, or poor overall physical condition.
USE OF THE WORLD HEALTH ORGANIZATIONS FRACTURE RISK ALGORITHM (FRAX)
To enable a practitioner to assess the 10-year probability of a hip fracture and the 10-year probability of a major osteoporotic fracture (defined as clinical vertebral, hip, forearm, or proximal humerus fracture), the Fracture Risk Assessment Tool [FRAX] was established by the World Health Organization in 2008. The FRAX was developed to provide general clinical guidance for treatment decisions. The FRAX takes into account femoral neck BMD and the following clinical risk factors: patient’s current age, sex, a prior osteoporotic fracture (including morphometric vertebral fracture), femoral neck BMD, weight (kg) or body mass index (kg/m2), use of oral glucocorticoids ≥5 mg/d of prednisone for ≥3 months duration (ever), the presence of rheumatoid arthritis, the presence of secondary osteoporosis, parental history of hip fracture, current smoking status, and alcohol intake (3 or more drinks per day).
The WHO algorithm used in this guide was calibrated to US fracture and mortality rates; therefore, the fracture risk figures herein are specific for the US population. There have been noted to be several limitations with the use of the FRAX in the United States: FRAX is intended for postmenopausal women and men aged 50 and older; it is not intended for use in younger adults or children. The FRAX tool has not been validated in patients currently or previously treated with pharmacotherapy for osteoporosis. In such patients, clinical judgment must be exercised in interpreting FRAX scores. In the absence of femoral neck BMD, total hip BMD may be substituted; however, use of BMD from non-hip sites in the algorithm is not recommended because such use has not been validated. The WHO determined that for many secondary causes of osteoporosis, fracture risk was mediated primarily through impact on BMD (255). For this reason, when T-scores are inserted into FRAX, the secondary osteoporosis button is automatically inactivated.
Despite these limitations as noted, it has been suggested that calculation of a FRAX score can supplant the need to do DEXA scans and to just use this score as a measure of osteoporosis risk in patients with IBD (256). This suggestion was based on a small retrospective study and needs prospective validation. Another study suggested that the clinical FRAX score alone seems to overestimate the risk of osteoporotic fracture in the population of patients with IBD that were studied (257). Recently, the FRAX tool was validated in the United Kingdom in a prospective cohort of 454,499 women aged 40–85 years and 424,336 men from 357 general practices and was found to predict the actual incidence of fractures. The FRAX predictions were similar to those observed in the cohort (258). There has been a recently updated version of the FRAX called the FRAXplus. FRAX is available in 86 models for 78 countries. FRAX has been criticized for several different reasons, but mostly because of the restriction in the number of risk factors that have been incorporated into the model. It was initially designed to be a simple tool, accessible, and easy to use in primary care. Thus, only a yes or no answer is accommodated for most questions in the tool. Thus, risk factors which are number-dependent or dose-dependent are not fully assessed. The new FRAXplus website has addressed some of the critiques of the initial FRAX (259). There have been other tools to assess risk including the Garan fracture calculator (260) and Qfracture (261).
Assessment for previous fractures
The decision as to whether treatment of osteoporosis should be initiated is not only based on the result of the BMD but also it is important to consider other risk factors for fracture which are independent of the BMD. If a patient has evidence of a previous low-impact fracture, this would represent a component of the fracture risk evaluation that is of paramount importance because low-impact (fragility) fractures convey the strongest risk factor for further fractures.
Routine assessment of spinal x-rays should not be initiated unless patients demonstrate height loss ≥4 cm compared with the reported height at age 20 years, height loss ≥2 cm compared with height measured during follow-up, or if they have symptoms such as back pain (262).
How frequently should BMD be assessed in individuals with IBD?
The factors which affect the determination of the optimal time interval for follow-up are a function of the DEXA machine precision and the expected rate of bone loss. The frequency of monitoring patients with abnormal BMD on therapy using DEXA scans is an area of controversy. Several physicians recommend DEXA scans be repeated at 1-to-2-year intervals to monitor specific therapeutic benefits on BMD; however, recent scientific data refute these suggestions. In general, the rate of change of BMD may be smaller than the measurement error of the DEXA scanner itself. Stated differently, repeat DEXA scans are unable to differentiate between a true increase in bone density or a variation in the measurement from the machine itself. Typically, BMD will change 1% annually, which is less than the error of a DEXA machine (typically this is in the range of 3%). In other words, changes of less than 2%–4% in the vertebrae and 3%–6% at the hip can be a consequence of the precision error of the method used.
A study (in patients without IBD) did however suggest that clinicians should monitor with DEXA approximately at 1 year of treatment in order to identify the substantial number of “real-world” patients who lose significant BMD despite treatment (263). They highlighted that those patients who lost bone density did have higher rates of bony fractures. It is uncertain whether this observed benefit is a consequence of other factors such as poor adherence to therapy, or underlying secondary causes of osteoporosis, or because of poor response to one of the bisphosphonates. Overall, approximately 10% of the patients started on oral bisphosphonate in clinical practice have a significant decrease in BMD on follow-up DEXA (264). Considering these recent findings, a repeat DEXA scan after initiation of therapy is appropriate within a minimum of 1 year.
Recommendation
Summary of evidence.
Since the original report on the effects of smoking on human health by the Surgeon General in January 1964, physicians have recommended smoking cessation to their patients. For CD, there are data to suggest that smoking is associated with (i) development of disease and (ii) disease progression with worse medical and surgical outcomes.
Development of disease
The first epidemiologic study to document the increased risk of CD associated with smoking came from the United Kingdom in 1984, where a population-based cohort of patients who smoked were shown to have a more than threefold increased risk for developing CD compared with healthy controls (RR 3.5, 1.8–6.6) (265). Another later population-based case–control study from New Zealand demonstrated a twofold increased risk for cigarette smoking at diagnosis (OR 1.99, 95% CI 1.48–2.68) (266). Finally, the Oxford Family Planning Association contraceptive study showed that the risk of developing CD in women was more than threefold higher in smokers than nonsmokers (267). In twin studies, Bridger found that in 23 dichotomous twin pairs, the smoker twin had CD in 91% of cases with an OR of 10.5 (95% CI 2.6–92) and in another more recent study, the smoking twin from discordant pairs demonstrated an OR of 2.9 (95% CI 1.2–7.1) (255). A meta-analysis by Mahid et al examined a total of 9 studies reporting on smoking and development of CD (268). They found a significant association between current smoking and the development of CD with an OR of 1.76 (95% CI 1.40–2.22) without significant heterogeneity.
Effect on disease course
Data from 1,420 incident patients between January 1977 and December 2008 found that smoking was associated with a change in disease behavior (P = 0.02), development of arthritis/arthropathy (P = 0.002), and need for steroids (P = 0.06) or thiopurine therapy (P = 0.038) (269). Data from a large Spanish national IBD registry (ENEIDA), including information regarding demographics, clinical characteristics, disease complications, therapeutic interventions, and smoking status demonstrated that in the time-dependent multivariate analysis, smokers were found to have a significantly decreased survival free of stricturing disease (HR 1.5, 1.18–1.90) or perianal complications (HR 1.50, 95% CI 1.01–1.46), and had a higher risk for requiring thiopurine therapy (HR 1.20, 95% CI 1.05–1.30) (270). In the follow-up TABACROHN study, this multicenter cross-sectional study included 1,170 patients with CD. Patients were classified as nonsmokers, current smokers, or former smokers according to their present smoking status. Smokers were more frequently under maintenance treatment when compared with nonsmokers. In addition, current smokers presented higher use of biologic drugs compared with nonsmokers. Tobacco exposure and a higher tobacco load were independent predictors of need for maintenance treatment and stenosing phenotype, respectively (271). In a recent meta-analysis, To et al (272) found in 33 studies a 56% higher risk of a flare of disease activity and a twofold risk of flare after surgery along with a need for first surgery (OR 1.68, 95% CI 1.33–2.12) and the need for second surgery (OR 2.17, 95% CI 1.63–2.89) in smokers.
Smoking also seems to adversely affect response to therapy for either CD or UC. Early reports of anti-TNF therapy found that 22% of smokers vs 74% of nonsmokers responded to episodic use of infliximab (273), and a prospective study of 74 patients given a single dose of infliximab found that at 4 weeks, smokers were less likely to have a response (OR 0.22, 95% CI 0.08–0.41) and a shorter duration of response than nonsmokers (274). In a retrospective study, patients with CD who continued to smoke required new courses of steroids and in multivariate analysis, smoking status was the only predictive factor of drug tolerance (275). In a retrospective study of 83 patients with CD who underwent endoscopic balloon dilation of an intestinal stricture, Gustavsson found among current smokers, 97% underwent another intervention compared with only 55% among never smokers (adjusted HR 2.50, 95% CI 1.14–5.50). After 5 years, the cumulative probability of a new intervention was 0.81 in smokers compared with 0.52 in never smokers, the difference 0.29 (95% CI 0.07–0.52) (276).
The positive effect of smoking cessation has also been demonstrated. Ryan et al (277) reported on the effect of smoking cessation on surgical outcomes with a favorable outcome in those quitting less likely to have undergone 1, 2, and 3 reoperations for recurrence at any site and were less likely to have undergone 1 reoperation for recurrent ileocecal CD than those who continued to smoke. In a controlled trial of the effect of smoking cessation on CD, repeated counseling to stop smoking, with easy access to a smoking cessation program, was given to 474 consecutive smokers with CD. Patients who quit smoking for more than 1 year (quitters) were included in a prospective follow-up study, which compared disease course and therapeutic needs with 2 control groups, continuing smokers, and nonsmokers, paired for age, sex, disease location, and activity. There were 59 quitters (12%). Predictors of quitting were the physician, previous intestinal surgery, high socioeconomic status, and in women, oral contraceptive use. During a median follow-up of 29 months (1–54 months), the risk of flare-up in quitters did not differ from that in nonsmokers and was less than in continuing smokers (P 278). There are several proposed biologic mechanisms as possible explanations for this association ranging from altered autophagy and consequent epithelial oxidative damage (279), genetics with altered IL23R SNPs (280), dysfunctional mononuclear cells (281), and altered bacterial microbiota profiles in smokers with CD (282).
Updated epidemiologic studies continue to demonstrate adverse effects on disease course in smokers. In a UK database study, smokers at CD diagnosis had significantly higher corticosteroid use (56 vs 47%, P 1 flare/year: 9 vs 6%, P P 283). In a retrospective study in India, while they did not find an association between need for biologics or surgery, they did find smokers were more likely to require hospitalization (aOR 2.59, 95% CI 1.22–5.49) (284). In South Korea, smoking was associated with a higher risk of developing CD (adjusted hazard ratio [aHR] 1.46, 95% CI 1.19–1.79) and elderly former smokers also had an increased risk for developing CD (aHR 1.68, 95% CI 1.22–2.30) (285).
Regarding recurrence after resection, a French nationwide study prospectively followed 289 patients with CD undergoing ileocecal resection; 70 (32%) were active smokers at time of surgery. Endoscopic recurrence occurred in 107 (47%) patients. In multivariate analysis, male sex (OR 2.48, 95% CI 1.40–4.46), active smoking at surgery (OR 2.65, 95% CI 1.44–4.97), and previous resection (OR 3.03, 95% CI 1.36–7.12) were associated with a higher risk of endoscopic recurrence (286). In a follow-up study, 346 patients undergoing ileocolonic resection, endoscopic recurrence rates of 57.6% were noted, and in multivariate analysis, absence of postoperative smoking (OR 0.60, 95% CI 0.40–0.91) was protective against endoscopic recurrence (287). A retrospective review of 2 specialist centers examined 290 patients undergoing ileocolic resection between 2000 and 2012. Kaplan-Meier survival analysis showed a significantly higher surgical recurrence rate for smokers vs nonsmokers (16/42 [38%] vs 3/31 [10%]; P = 0.02; risk ratio 3.9, 95% CI 1–12) (288). In a follow up meta-analysis to examine the association between smoking and induction of clinical response or remission with anti-TNF therapy, 18 observational studies and three RCTs were included (289). Current smokers and nonsmokers (never or former) had similar rates of clinical response (observational studies: RR 0.96, 95% CI 0.88–1.05; RCTs: RR 1.09, 95% CI 0.84–1.41). When restricted to studies clearly defining the smoking exposure, smokers treated with anti-TNF were less likely to achieve clinical response than nonsmokers (smokers defined as having ≥5 cigarettes per day for ≥6 months) (RR 0.63, 95% CI 0.48–0.83); lifetime never smokers vs ever smokers excluding former smokers was protective of clinical response (RR 0.81, 95% CI 0.71–0.93). Current smokers were also less likely to achieve clinical remission in observational studies (RR 0.75, 95% CI 0.57–0.98), although this association was not seen in RCTs (RR 1.04, 95% CI 0.89–1.21).
In the first study to describe the role of cigarette smoke in colorectal neoplasia (CRN) development in IBD, investigators examined 1,386 patients with IBD with previous biopsies (290). Clinical factors and cigarette smoke were evaluated. Cox-regression modeling was used to estimate the effect of cigarette smoke and its additive effect within the current risk stratification for prediction of CRN. One hundred fifty-three (11.5%) patients developed CRN. Previously described risk factors including first-degree family member with CRN in CD (P = 0.001), presence of postinflammatory polyps in UC (P = 0.005), were replicated. Former smoking increased risk of CRN in UC (HR 1.73, 95% CI 1.05–2.85), whereas passive smoke exposure yielded no effect. For CD, active smoking (HR 2.20, 95% CI 1.02–4.76) and passive smoke exposure (HR 1.87, 95% CI 1.09–3.20) significantly increased CRN risk.
CONCLUSION
Patients with IBD often consider their gastroenterologist to be the primary provider of care. Because more than 70% of patients with IBD will at some time be on medications that will affect the immune system, it is essential that the gastroenterology team promote vaccinations and other health maintenance activities. Referral to dermatology, endocrinology, gynecology, primary care, and psychiatry may be necessary on a case-by-case basis. Coordination between the gastroenterology team and other providers is crucial for improving the quality of care that we provide to our patients living with IBD.
CONFLICTS OF INTEREST
Guarantor of the article: Francis A. Farraye, MD, MSc, MACG
Specific author contributions: F.A.F., G.Y.M., G.R.L., F.C., and S.K.: contributed to planning, literature review and analysis, guidance statement determination, writing, and final revision of the manuscript. E.L.B. and B.N.L. provided methodology expertise and reviewed the evidence for GRADE assignments.
Financial support: None to report.
Potential competing interests: F.A.F. has served on advisory boards for AbbVie, Avalo Therapeutics, Bausch, Bristol Myers Squibb, Braintree Labs, Eli Lilly (DSMB), Fresenius Kabi, GI Reviewers, GSK, IBD Educational Group, Iterative Health, Janssen, Pharmacosmos, Pfizer, Sandoz Immunology and Viatris. G.Y.M. has served on advisory boards for AbbVie, Arena, Bioquest, Boomerang, Bristol-Myers Squibb, Boehringer-Ingelheim, Celgene, Diasorin, Ferring, Fresenius Kabi, Gilead, Iterative Scopes, Janssen, Lilly, OptionCare, Pfizer, Rippling, Samsung Bioepis, Shield Therapeutics, Takeda, Techlab, Verantos, Viatris; he has received research support from NIDDK, Helmsley Charitable Trust, Crohns and Colitis Foundation, Pfizer; he has stocjk options in Dieta and Oshi Health; he has received research funding from Pfizer; and he is a speaker for AbbVie and Janssen. G.R.L. has served as a consultant for: Abbvie, American Regent, Bristol Myers Squibb, Celgene, Eli Lilly, Endo Pharmaceuticals, Ferring, Gilead, Janssen Orthobiotech, Kabi Fresenius (Health and Wellness Partners), MedEd Consultants, Pharmacosmos, Pfizer, Prometheus Laboratories, Sandoz, takeda and UCB; he has participated in CME programs supported by: Abbvie, Allergan, American Gastroenterological Association, American Regent, Annenberg Center for Health Sciences, Boehringer Ingelheim, Bristol Myers Squibb, Eli Lilly, Exact Sciences, Ferring, IBD Horizons, Ironwood, Janssen, MedEd Consultants, Pennsylvania Society of Gastroenterology, Sanofi, Salix, Seres Pharmaceuticals, Takeda, Vindico; other finances received include Eli Lilly for DSMB (Honorarium for Data Safety Monitoring Board), Gastro-Hep Communications (Honorarium for executive editor), Janssen Orthobotech (Finances to Fund GI IBD Fellowship), Pfizer (Finances to Fund GI IBD Fellowship), Professional Communications—textbook royalty, Springer Science and Business Media—Editor (Honorarium), Up To Date—Walters Kluwer—Author (Honorarium); and he has received research support from Takeda, UCB, Bristol Myers Squibb, Janssen Orthobiotech. E.B. has been a consultant to AbbVie, Boomerang, Eli Lilly, Pfizer, and Takeda. B.N.L. has served on the advisory board of Johnson & Johnson. F.C. received research support from GSK, Takeda Pharmaceuticals, Janssen, and Novavax, and has been a consultant for Takeda, Arena Pharmaceuticals, GSK, and Celgene. S.K. has been a consultant to AbbVie, Boehringer Ingelheim, Bristol Myers Squibb, Celltrion, Fresenius Kabi, Janssen, Lilly, Takeda; and serves as IBD Section Editor for UptoDate.
REFERENCES







