INTRODUCTION
Crohn’s disease (CD) has been increasing in incidence and prevalence worldwide. At the same time, the number of therapeutic options is rapidly increasing. The purpose of this guideline was to review CD clinical features and natural history, diagnostics, and therapeutic interventions.
To prepare this guideline, literature searches on the different areas were conducted using Ovid MEDLINE from 1946 to 2025, EMBASE from 1988 to 2025, and SCOPUS from 1980 to 2025. The major terms that were searched were CD, inflammatory bowel diseases (IBDs), regional ileitis, and regional enteritis. These were translated into EMTREE controlled vocabulary as enteritis and CD. The remainder of the search included key words related to the subject area that included clinical features, natural history, diagnosis, biomarkers, treatment, and therapy. For each of the therapeutic sections, key words included the individual drug names. The results used for analysis were limited to primary clinical trials, meta-analyses, systematic reviews, and prior guidelines. Where there were limited data, observational data were used. In areas where data were limited, and Grading of Recommendations Assessment, Development, and Evaluation (GRADE) was not feasible, key concept statements were developed from expert opinion of the literature.
Where possible, the GRADE process was used to evaluate the quality of supporting evidence. A strong recommendation is made when the benefits or desirable effects of an intervention clearly outweigh the negatives or undesirable effects and/or the result of no action. The term conditional is used when some uncertainty remains regarding the balance of benefits and potential harms, either because of low-quality evidence or because of a suggested balance between desirable and undesirable effects. The quality of the evidence is graded from high to low, where high-quality evidence indicates that the authors are very confident that the true effect lies close to that of the estimate of the effect. Moderate-quality evidence is associated with moderate confidence in the effect estimate, although further research would be likely to have an impact on the confidence of the estimate. Low-quality evidence indicates limited confidence in the estimate, and thus, the true effect could differ from the estimate of the effect. Very low-quality evidence indicates very little confidence in the effect estimate and that the true effect may be substantially different than the estimate of effect (1–3).
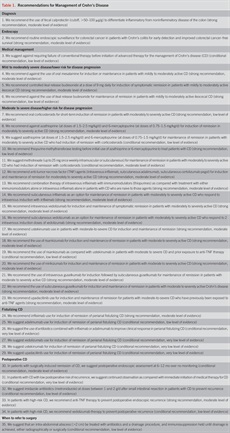
We preferentially used meta-analyses or systematic reviews when available, followed by clinical trials and retrospective cohort studies. The GRADE recommendations statements from this guideline are in Table 1. Summary Key Concept statements, which do not have associated evidence-based ratings, are in Table 2.
Recommendations for Management of Crohn’s Disease
Key concepts
CLINICAL FEATURES
Key concept
The most common symptom of CD is chronic diarrhea, but some patients may not experience this symptom (4). Abdominal pain, often localized to the right lower quadrant of the abdomen and worsened postprandially, is common. Fatigue is also a very prevalent symptom in CD and is believed to arise from several factors including inflammation itself, anemia, or various vitamin and mineral deficiencies. Some patients will present with constitutional signs or symptoms including fever, weight loss, or, in the case of younger patients, growth failure.
Key concept
The clinician must integrate multiple streams of information, including history and physical, laboratory tests, endoscopy results, pathology findings, and radiographic tests, to arrive at a clinical diagnosis of CD. In general, it is the presence of chronic intestinal inflammation that solidifies a diagnosis of CD. Distinguishing CD from ulcerative colitis (UC) can be challenging when inflammation is confined to the colon, but clues to the diagnosis include discontinuous involvement with skip areas, sparing of the rectum, deep/linear/serpiginous ulcers of the colon, strictures, fistulas, or granulomatous inflammation. Granulomas are present on biopsy in only a minority of patients. The presence of ileitis in a patient with extensive colitis (backwash ileitis) can also make determination of the IBD subtype challenging.
Key concept
A systematic review of population-based cohort studies of adult patients with CD identified an increased risk of bone fractures (30%–40% elevation in risk) and thromboembolism (3-fold higher risk) (5). A variety of extraintestinal manifestations, including PSC, ankylosing spondylitis, uveitis, pyoderma gangrenosum, and erythema nodosum, have been observed in patients with CD. Moreover, there are weak associations between CD and other immune-mediated conditions, such as asthma, psoriasis, rheumatoid arthritis, and multiple sclerosis.
NATURAL HISTORY
Key concept
The chronic intestinal inflammation that occurs in CD can lead to the development over time of intestinal complications such as strictures, fistulas, and abscesses. These complications can lead to inhibition of intestinal function or to surgery that itself can result in some morbidity and loss of intestinal function. A scoring system, the Léman index, has been created to quantify the degree of bowel damage incurred by intestinal complications and subsequent surgery (6). This index has been shown to be reproducible and internally consistent, and median index scores rise with disease duration (7). In a population-based cohort study from Olmsted County, Minnesota, of 147 patients with CD who had undergone at least 1 bowel resection (median follow-up per patient, 13.6 years), the median cumulative length of bowel resected was 64 cm, and the median rate of bowel resection was 4.2 cm annually (8).
Key concept
Population-based studies from Norway and Minnesota suggest that CD presents with ileal, ileocolonic, or colonic disease in roughly one-third of patients each, with up to a quarter also having upper gastrointestinal (GI) involvement and that only a small minority of patients (6%–14%) will have a change in disease location over time (9–11).
Key concepts
Multiple population-based cohorts of CD have demonstrated that most of the patients (between 56% and 81%) have inflammatory disease behavior at diagnosis, whereas between 5% and 25% each present with stricturing or penetrating disease behavior (10). A population-based study from Olmsted County showed that the cumulative risk of developing an intestinal complication among those presenting with inflammatory behavior was 51% at 20 years after diagnosis (12). Multivariate analysis demonstrated that ileal, ileocolonic, or upper GI involvement, relative to colonic involvement, was significantly associated with faster time to the development of intestinal complications. Colonic disease was also found to be protective against the progression to complications in the multicentric European Epi-IBD cohort (13). Additional risk factors associated with a more severe CD course include younger age at diagnosis, extensive luminal involvement, perianal disease, and severe rectal disease (14,15). Awareness of these clinical features at the time of presentation is essential for early initiation of medical and/or surgical therapies.
Key concepts
Several studies illustrate the disconnect between symptoms and inflammation. For example, in a prospective study of 142 patients treated with prednisolone for 3–7 weeks, there was no correlation between Crohn’s Disease Activity Index (CDAI) scores and Crohn’s Disease Endoscopic Index of Severity scores (16). In a cross-sectional study of 164 patients with CD, not only did CDAI scores not correlate with Simple Endoscopic Score for Crohn’s Disease (SES-CD) scores, they also did not correlate with serum C-reactive protein (CRP), fecal calprotectin (FC), and fecal lactoferrin (17).
Key concept
In population-based cohorts, the frequency of perianal fistulas is between 10% and 26%, and the cumulative risk was 26% at 20 years after diagnosis in 1 cohort (10,18,19). Perianal disease at diagnosis may indicate a more severe clinical course of CD. More recent population-based studies suggest that the cumulative incidence of perianal disease may be decreasing (20). A recent systematic review of population-based cohorts estimated the prevalence of perianal involvement in CD to be 18.7% and that the 10-year progression to perianal CD was 18.9% (21).
The onset of perianal CD may occur before the onset of luminal CD. In a recent systematic review and meta-analysis, it was reported that 3.8% (based on 5 studies, 95% confidence interval [CI] 1.9%–7.3%) of patients with CD developed perianal disease before luminal CD diagnosis (21). In a population cohort study form New Zealand which evaluated 715 patients with CD over with median follow-up after CD diagnosis was 9 years, it was observed that perianal lesions can be the first manifestation preceding the diagnosis of CD by > 6 months in 17% patients; in 27% perianal disease presents from 6 months before to 6 months after the diagnosis of CD, whereas perianal disease is first observed >6 months after CD diagnosis in the remaining 56% (22). However, it remains unclear whether all patients in this study underwent a thorough assessment for luminal disease by means of cross-sectional imaging of the abdomen or capsule endoscopy.
Key concept
A population-based study from Olmsted County, Minnesota, modeled the lifetime course of CD in various disease states using a Markov model; the model was unique in that the transition probabilities between disease states were derived by mapping disease states to the actual chronological history of each patient (23). Over the lifetime disease course, a representative patient spent 24% of the duration of their disease in a state of medical remission, 27% in mild disease, 1% in severe drug-responsive disease, 4% in severe drug-dependent disease, 2% in severe drug-refractory disease, 1% in surgery, and 41% in postsurgical remission. In the 1962–1987 Copenhagen County cohort, within the first year after diagnosis, the proportions of patients with high activity, low activity, and clinical remission were 80%, 15%, and 5%, respectively (24). However, after the first year through 25 years, a decreasing proportion of high activity (30%), increasing proportion of remission (55%), and stable proportion of mild activity (15%) were observed.
Key concept
Population-based studies from Denmark and Minnesota suggest that between 43% and 56% of patients with CD received corticosteroids in the prebiologic era and that over half of these patients were steroid-dependent, steroid-refractory, or required surgical resection within the subsequent year (25,26). In a study from Minnesota in the biologic era, 1-year outcomes after the use of corticosteroids included prolonged remission in 60%, steroid dependency in only 21%, and resection in 19% (27).
Key concept
An older Copenhagen County study suggested that 83% of patients were hospitalized within 1 year of diagnosis, and the annual rate of hospitalization thereafter was approximately 20% (25). Up to 70% of Olmsted County patients were hospitalized at least once, and the cumulative risk of hospitalization in the prebiologic era was 62% at 10 years. The annual rate of hospitalization was highest in the first year after diagnosis (19). A recent systematic review and meta-analysis of population-based cohorts of CD estimated a cumulative risk of hospitalization of 44%–49% at 5 years and up to 59%–72% at 10 years (28).
Key concept
In a systematic review of 30 publications examining major abdominal surgical risk in CD, the cumulative incidence of surgery was 46.6% at 10 years and that this risk was reported to be lower, under 40%, among patients who had been diagnosed after 1980 (29). Another systematic review examined the risk of a second resection among those patients with CD who had undergone a first resection, and this was estimated to be 35% at 10 years overall, but significantly lower among those patients diagnosed after 1980 (30). A recent systematic review and meta-analysis of population-based cohorts estimated that the cumulative incidence of surgery in CD had decreased in relative terms by 45%–50% in the postbiologic era (for example, the 10-year risk of surgery decreased from 46.5% before 2000 to 26.2% after 2000) (31).
Key concept
Among patients with CD who undergo major abdominal surgery, the 5-year cumulative risk of clinical recurrence is 40%–50% (32,33). The risk of endoscopic recurrence approaches 90%. Risk factors for recurrent CD postoperatively include cigarette smoking, shorter duration of disease before operation, more than 1 resection, and penetrating complications. In a systematic review of 37 studies (mixture of cohort studies and randomized trials), with a median follow-up ranging from 72 to 162 weeks, the pooled crude endoscopic recurrence rate was 52%–57%, and the pooled crude clinical recurrence rate was 25%–31% (34).
Key concept
A 2007 meta-analysis of 13 studies of CD mortality yielded a pooled standardized mortality ratio of 1.5 (35). There was a nonsignificant trend for decreased mortality in more recent studies. In a 2013 meta-analysis, the pooled standardized mortality ratio for CD was 1.46 and slightly lower at 1.38 when restricted to population-based and inception studies. This study confirmed a previously noted association between CD and increased mortality from respiratory disease (36). Several studies have demonstrated an association between current use of corticosteroids and increased mortality in CD (37,38). A large Danish study showed no change in relative mortality in CD between 1982 and 2010, roughly 50% higher than the general population (39). Mortality was 25% higher than expected among patients with CD from Olmsted County, and this was largely driven by those diagnosed before 1980 (40).
INTESTINAL MALIGNANCY
Key concept
Patients with CD with colitis are at increased risk of CRC (41). Similar to UC, risk factors for CRC include duration of CD, PSC, and family history of CRC.
Key concept
The relative risk (RR) of small bowel adenocarcinoma in patients with CD is markedly elevated (at least 18-fold), although the absolute risk remains low, in the order of 0.3 cases per 1,000 patient-years (42). The increased risk is believed to arise from longstanding chronic inflammation.
DIAGNOSIS
The diagnosis of CD is based on a combination of clinical presentation and endoscopic, radiologic, histologic, and pathologic findings that demonstrate some degree of focal, asymmetric, transmural granulomatous inflammation of the luminal GI tract. Laboratory testing is complementary in assessing disease severity and complications of disease. There is no single laboratory test that can make an unequivocal diagnosis of CD. The sequence of testing is dependent on presenting clinical features.
Symptom assessment
Evaluation of clinical disease activity should include assessment of stool frequency and consistency, the presence of abdominal pain, systemic signs of inflammation (e.g., fever, weight loss, tachycardia, and anemia), and extraintestinal manifestations of CD. In addition, other clinical features may include obstructive symptoms, food aversion, and dietary changes. Rectal pain or defecatory issues may be associated with perianal CD.
However, other conditions may present with symptoms indistinguishable from active luminal CD. Therefore, an essential part of clinical evaluation is to determine whether presenting symptoms are due to CD vs other conditions, such as bile salt diarrhea, intestinal infection, small intestinal bacterial overgrowth (especially for patients with an ileocolonic resection or known intestinal strictures), bypass from a fistula, dietary intolerances, disorders of the gut-brain interaction, anorectal sphincter dysfunction, medication-related adverse event, or potential mimickers of CD (e.g., endometriosis, tuberculosis). When diagnostic uncertainty is present because of clinical symptoms, it is recommended to confirm disease activity through imaging and/or endoscopic assessments. In individuals without any observable mucosal inflammation or ulceration, consideration should be given to the potential differential diagnostic possibilities.
Routine laboratory investigation
Key concepts
Recommendation
Patients presenting with suspected CD often will show laboratory evidence of inflammatory activity. Anemia and an elevated platelet count are the most common changes seen in the complete blood count (43,44). CRP is an acute phase reactant produced by the liver in the presence of inflammation. It is elevated in a subset of patients with CD. It has a short half-life of 19 hours. Because of its short half-life, serum concentrations decrease quickly, making CRP a useful marker to detect and monitor inflammation (see later section) (45,46). Erythrocyte sedimentation rate (ESR) may be useful in an individual patient, but it is not predictive of IBD and does not discriminate patients with IBD from those with IBS or healthy controls (47). Up to 40% of patients with IBD with mild inflammation may have a normal CRP and ESR, limiting the usefulness of these markers in monitoring some patients (48). Signs and symptoms of bowel inflammation related to IBD overlap with those of infectious enteritis and colitis. Stool studies for fecal pathogens and Clostridioides difficile will help direct diagnosis and management. FC is a calcium-binding protein derived from neutrophils and plays a role in the regulation of inflammation. It is a sensitive marker of intestinal inflammation. Other proteins in the stool derived from neutrophils include lactoferrin, lysozyme, and elastase. In an inflamed bowel, these proteins may be released into the stool. Measurements of FC serve as noninvasive markers of intestinal inflammation and may be useful in differentiating patients with IBD from those with irritable bowel syndrome (49). A recent meta-analysis found that a FC level of 50 μg/g had a sensitivity of 88% and a specificity of 72% in distinguishing IBD from functional GI disease (50). Other studies suggest cutoff values ranging from 50 to 100 μg/g (51,52). Fecal markers may also be useful in monitoring disease activity and response to treatment (53).
Genetic testing
Key concepts
CD is a heterogeneous disease with complex interactions between genetics, environmental exposures, and the intestinal microbiome. To date, there are over 200 genetic loci associated with IBD and greater than 71 CD susceptibility loci that have been identified through large-scale genome-wide association studies (54–56). As more genetically diverse populations are studied, this is likely to expand. Examples of single-nucleotide polymorphisms that confer susceptibility to CD include sequences in the nucleotide-binding oligomerization domain-containing protein 2 (NOD2) gene, the interleukin (IL)-23 receptor gene, and the autophagy-related 16-like 1 gene (57). These genes play a role in innate immunity and regulation of the epithelial barrier (58). These susceptibility variants are biologically important in understanding the pathophysiology of CD, but there is no single variant that has a high enough frequency in the CD population to make it diagnostically useful. There is significant variation in the prevalence of susceptibility genes between various racial/ethnic groups—for example, NOD2 and IL23R variants are very uncommon in East Asian populations (54). There are genetic variants that are associated with disease phenotype. NOD2 variants are predictors of a more complicated disease behavior including ileal involvement, stenosis, and penetrating disease behaviors and the need for surgery (59). These variants are also associated with early disease onset (60). IL-12B variants are associated with the need for early surgery (61). NOD2 testing is commercially available for 3 of the most common variants seen in CD. Although identification of these variants may identify patients who are likely to have more aggressive CD, this laboratory test has not been routinely used clinically and remains a research tool. Ultimately, we may be able to use genetic testing to characterize patient’s disease behavior and guide early therapy (62). Other potential uses of genetic testing include predicting both responses to and adverse events related to drug therapy for IBD. NUDT15 and TPMT variants are associated with thiopurine-induced leukopenia (63). The HLADQA1*05 and the HLA-DRB1*03 haplotypes have been associated with increasing immunogenicity to tumor necrosis factor (TNF) antagonists (64–67).
Serologic markers of IBD
Key concept
Because of the heterogeneous nature of IBD, there has been extensive research directed toward finding immunologic markers that would assist in disease diagnosis. These studies have focused on antibodies to microbial antigens and autoantibodies (68–73). Anti-glycan antibodies are more prevalent in CD than in UC but have a low sensitivity, making their use in diagnosis less helpful (73). Tests have been developed that use a combination of serologic, genetic, and inflammatory markers to try to improve diagnostic efficacy; however, this combination of markers has not improved serology measurements usefulness as a screening tool (74).
Endoscopy: colonoscopy
Key concepts
Colonoscopy with intubation of the terminal ileum and biopsy of endoscopically involved and uninvolved mucosa are recommended as part of the initial evaluation of patients with suspected IBD. Over 80% of patients with IBD will have mucosal involvement within the reach of the colonoscope. Ileal intubation rates are as high as 80%–97% in patients in whom the cecum is reached (75). Computed tomography enterography (CTE) and magnetic resonance enterography (MRE) examinations of the terminal ileum may both over- and under-represent disease of the ileum but are useful for detection of more proximal disease. Direct evaluation of the ileum will complement radiographic findings in the diagnosis of CD. Mucosal changes suggestive of CD include mucosal nodularity, edema, ulcerations, friability, and stenosis (75–77). Classical granulomatous inflammation is seen in a minority of patients (up to 33%) with CD and is helpful but not required for diagnosis. Disease distribution of endoscopic and histologic findings is important to document at the time of diagnosis because this has implications on screening for CRC, disease prognosis, and in the future—affect therapeutic decision-making. Attempts to quantify the distribution and severity of mucosal involvement of the colon and the ileum in patients with CD have led to the development of multiple endoscopic scoring systems, of which the SES-CD is the simplest to use (78,79). Studies using central readers have shown excellent intrarater and inter-rater reliability (80). This tool is available in many endoscopic documentation programs and may allow for serial assessment of the mucosa during therapeutic interventions in CD (see later section).
Colonoscopy for CRC surveillance
Recommendation
Most surveillance guidelines have been adapted from UC practice guidelines. Recently, a study of 23,751 colonoscopies in patients with IBD demonstrated that the rate of progression of dysplasia was similar in patients with UC and CD. These findings support that surveillance strategies should be similar for both UC and CD (81). Surveillance colonoscopy is suggested for patients who have a minimum of 8 years of disease with involvement of more than 30% of their colon. The risk of neoplasia in Crohn’s colitis increases with both the duration and the extent of disease (82). PSC and diagnosis of CD before the age of 40 years are also associated with increased risk of both CRC incidence and mortality (83,84). CRC surveillance has been shown to increase detection of early CRC and lead to decreased CRC mortality (85). Individuals with PSC should initiate surveillance colonoscopy at the time of their diagnosis regardless of disease distribution. The incidence of small bowel cancer is also increased in CD compared with the non-IBD population; however, routine surveillance is not currently recommended. There should be a high index of suspicion for small bowel cancer in a stable patient with small bowel CD who has an abrupt change in symptoms.
Key concept
CD of the upper GI tract is often underestimated, with most studies in adults suggesting that the range is 0.3%–5% (86,87). However, data from prospective studies suggest up to 16% of patients with CD have endoscopic and histologic changes of upper GI CD with only 37% of patients exhibiting upper GI symptoms at the time of evaluation (88). Routine endoscopic evaluation in asymptomatic patients with CD is associated with mild endoscopically visible inflammation in up to 64% of patients and histologic inflammation in up to 70% of patients (89). These studies have been performed predominantly in children. Despite these findings, there does not seem to be any clinical significance related to these mild changes (90). Endoscopic features suggestive of CD includes mucosal nodularity, ulceration (both aphthous and linear ulcerations), antral thickening, and duodenal strictures (91). Histologic changes include granulomatous inflammation, focal cryptitis of the duodenum, and focally enhanced gastritis (88,92).
Video capsule endoscopy
Key concepts
Small bowel capsule endoscopy allows for direct visualization of the mucosa of the small intestine. Isolated small bowel involvement may be seen in up to 30% of patients with CD, making it more challenging to diagnose with routine small bowel imaging techniques (93). Several meta-analyses have examined the diagnostic yield of capsule endoscopy in the evaluation of patients with suspected CD. Capsule endoscopy is superior to small bowel barium studies, CTE, and ileocolonoscopy in patients with suspected CD, with incremental yield of diagnosis of 32%, 47%, and 22%, respectively (93). Capsules with a panoramic 344o viewing area may improve complete mucosal visualization in patients with suspected CD (94). However, some studies have questioned the specificity of capsule endoscopy findings for CD, and to date, there is no consensus as to exactly which capsule endoscopy findings constitute a diagnosis of CD (95). The Lewis score is a scoring system based on the evaluation of 3 endoscopic parameters: villous appearance, ulcers, and strictures. The scoring system is incorporated into the software platform of some endoscopy capsules and assists in the quantification of small bowel inflammatory burden and diagnosis of CD (96). Capsule endoscopy has a high negative predictive value of 96% (97). The capsule retention rate in patients with suspected CD is 0%–5.4% and higher in those with known CD (98). Use of a patency capsule or small bowel imaging before video capsule endoscopy will reduce the risk of retention of the standard video capsule (99–102). A failed patency capsule study has also been shown to be associated with worse long-term clinical outcomes as compared with successful passage of the PC regardless of CD phenotype (103). Capsule endoscopy may also identify a site for directed biopsy to obtain tissue to establish a diagnosis of CD.
Imaging studies
Key concepts
The small bowel is one of the most common areas affected by inflammation in patients with CD. Much of the inflammation is beyond the reach of standard endoscopic evaluation. In up to 50% of patients with active small bowel disease, inflammation may skip the terminal ileum or be intramural and not detected by ileocolonoscopy (104). Complications of CD such as stricturing disease and enteric fistulas are best identified using small bowel imaging techniques. CTE has a reported sensitivity as high as 90% in detecting lesions associated with CD (95,105). The sensitivity for detecting active small bowel CD in 1 comparison study was only 65% with small bowel follow-through compared with 83% with CTE (95). In studies comparing capsule endoscopy with small bowel follow-through, there have been instances of patients with a normal small bowel follow-through showing both mucosal disease (20%) and stricturing disease (6%) on a capsule endoscopy (106). CTE features such as mucosal enhancement, mesenteric hypervascularity, and mesenteric fat stranding are all suggestive of active inflammation (107). MRE has similar sensitivity to CTE with wall enhancement, mucosal lesions, and T2 hypersensitivity as suggestive of intestinal inflammation (108). Studies with CT and MRE in patients with negative ileoscopy and biopsy that show unequivocal inflammation are associated with disease progression in 67% of patients (109). Inflammation scoring systems have been developed to provide quantification of the degree of inflammation. This may allow for assessment of treatment effects in serial examinations (110). Improvement in radiologic parameters for CTE and MRE with medical therapy is associated with a better clinical outcome regarding hospitalization, surgery, and corticosteroid use in patients with small bowel CD (111). The need for sequential imaging exams may be higher in young patients, patients with upper GI disease, those with penetrating disease, and patients who require steroids, biologics, and surgery. The need for repeated CTE studies over time leads to levels of diagnostic radiation exposure that theoretically might increase cancer risk (112,113). In these patients, MRE is preferred. Techniques to reduce dose of radiation exposure during diagnostic CT scanning have been implemented and currently being refined using changes in both software and hardware to maintain image quality with decreased radiation dosing. How this will alter the use of CTE is not known (114). IUS has been used in the management of CD in Europe for over a decade. Recently, there has been growing interest in its use and training in the United Sates. IUS enables real-time imaging to the intestinal wall, mesentery, and adjacent lymph nodes. Regarding diagnosis, point-of-care IUS can help identify bowel wall changes, potentially facilitating early referral for definitive diagnostic studies (115).
Key concept
Approximately 25% of patients with CD will develop a perirectal complication of their disease including fistula formation and/or perirectal abscess. With standard medical therapy, there is a high relapse rate of fistulous drainage. Imaging of the perianal area allows for identification of disease that requires surgical intervention to help with healing as well identify and classify all of the disease that is present premedical and postmedical therapy (116). Comparison studies have shown EUS to have greater than 90% accuracy in diagnosis of perianal fistulizing disease (117). Serial EUS examinations may be used to help guide therapeutic intervention in patients with fistulizing CD including seton removal and discontinuation of medical therapy (118,119). Magnetic resonance imaging of the pelvis has comparable accuracy (116,120). Scoring systems looking at disease activity and fibrosis have been developed and play a role in predicting treatment outcomes in perianal fistulizing disease (121).
Key concept
CTE and MRE both have an accuracy of greater than 90% in the detection of abscesses pre-operatively (122). Recently studies have shown that IUS is useful and accurate for the diagnosis of intra-abdominal complications of CD and can be used as a noninvasive, point-of-care evaluation in the appropriate clinical setting (123). CT can be used to help direct abscess drainage preoperatively which may lead to a lower rate of postdrainage complications (124).
Disease modifiers
Key concept
NSAIDs may cause damage to the small intestine distal to the duodenum resulting in mucosal ulcerations, erosions, and webs. Mucosal permeability is increased with NSAID therapy, leading to increased exposure to luminal toxins and antigens (125). In a comparison study of acetaminophen, naproxen, nabumetone, nimesulide, and aspirin, there was a 17%–28% relapse rate of quiescent IBD within 9 days of therapy with the nonselective NSAIDs (naproxen and nabumetone) (126). Recent NSAID use has been associated with an increased risk of emergency admission to the hospital for patients with IBD (127,128). However, in a large study of Veterans Association patients, the association between NSAID use and IBD flares was believed to reflect residual bias rather than a true causal association. When evaluating patients with both NSAID use and IBD, there were similar rates of disease activity pre-exposure as postexposure (129). In a systematic review and meta-analysis of studies with a low risk of bias, NSAID use was associated with an increased risk of CD exacerbation (130). Selective cyclooxygenase-2 inhibitors in short-term therapy have not been shown to exacerbate UC, but similar studies have not been performed in CD (131).
Key concept
Cigarette smoking has been shown in multiple clinical situations to have an adverse effect on the natural history of CD. There is an increased rate of surgical intervention, incidence of IBD hospitalizations, and peripheral arthritis in patients with CD who smoke as compared with nonsmokers (132,133). Active smoking has been associated with a penetrating phenotype in CD and increased risk of relapse with anti-TNF discontinuation (134). However, patients with CD who stop smoking have fewer disease flares and decreased need for corticosteroids and immunomodulatory therapy (135). Because cigarette smoking is a potentially modifiable variable affecting the clinical course of CD, current smokers should be counseled regarding risks of ongoing cigarette use and provided with smoking cessation resources (136).
Key concept
Many patients associate psychosocial stressors with increased CD symptoms. There is a high prevalence of anxiety and depression among patients with IBD with up to one-third of patients reporting anxiety and a quarter of patients with depressive symptoms (137). These comorbidities are associated with increased health care utilization including emergency department visits, hospitalizations, and treatment escalations (138–140). The psychosocial stressors affect CD management, likelihood of disease control, and quality of life. Multiple studies demonstrate a strong relationship between depression and anxiety with IBD symptoms and with increased risks of disability (125,141–144). Screening for anxiety and depression is an important preventive care measure for patients with IBD alongside using available resources for psychosocial support (136).
MANAGEMENT OF DISEASE
General principles
Management recommendations for patients with CD are based on disease location, severity, presence of disease-associated complications including extraintestinal manifestations, and factors affecting future prognosis. The anatomic distribution of disease is important primarily for medications with targeted delivery systems, such as enteric-coated budesonide. For all other agents (i.e., systemic corticosteroids, thiopurines, methotrexate, biologics, and oral small molecules), therapeutic activity against CD is believed to occur throughout the entire GI tract.
Therapeutic approaches are individualized with the composite goal of achieving clinical and endoscopic remission without significant adverse effects of treatment (145). CD treatment should be considered as a sequential continuum to treat acute disease or induce clinical remission and then to maintain response/remission with overall improvements in quality of life.
Objective evaluation by endoscopic, sonographic, or cross-sectional imaging is recommended to confirm the subjective improvement of symptoms. In general, clinical evidence of improvement should be evident within 2–4 weeks, and the maximal improvement should occur by 12–16 weeks. Patients achieving response or remission are then transitioned to appropriate steroid-sparing maintenance therapy. Patients with continued symptoms after induction warrant assessment to determine whether medication optimization, addition of other agents or transition to a different treatment strategy, either medical or surgical, according to their clinical status, disease activity, extent, and behavior is warranted.
For patients with continued active symptoms despite optimized therapy, evaluation with an objective study such as IUS, cross-sectional imaging (CTE or MRE), or endoscopy (e.g., ileocolonoscopy) is recommended to determine whether active disease is still present. While biomarkers of disease activity can be assessed (e.g., CRP, FC), these should not exclusively serve as a treatment endpoint because normalization of the biomarker may occur in the presence of active mucosal inflammation/ulceration. In addition, mimickers of active IBD such as C. difficile infections, cytomegalovirus infection, and medication-related adverse effects should be excluded. Patients with IBD have a higher carriage rate of toxigenic C. difficile as compared with controls (146,147). In patients who have an increase in symptoms of diarrhea after antibiotic therapy, concurrent C. difficile infection should be considered and evaluated. The risks of C. difficile infection may be up to 5-fold higher among patients with IBD, particularly those with additional risk factors such as corticosteroid use, anti-TNF use, hospitalization, or other comorbidities (148).
Therapeutic drug monitoring has become very common in the management of CD (149), especially among patients who initially responded to anti-TNF therapy but then developed loss of clinical response (secondary loss of response), and this approach has been endorsed by several national and international groups (150–153). If active CD is documented for persons receiving anti-TNF therapies, then assessment of anti-TNF drug levels and antidrug antibodies (therapeutic drug monitoring) should be considered. There can be 3 different scenarios explaining biologic failure: mechanistic failure, immune-mediated drug failure, and finally non–immune-mediated drug failure. Individuals who have therapeutic drug levels and no antibodies with the presence of active mucosal ulceration are considered to have mechanistic failure and a medication within another class and mechanism of action should be considered (e.g., in a patient on anti-TNF therapy with active inflammation, consideration of anti-IL or anti-integrin therapy). Non–immune-mediated pharmacokinetic mechanisms occur when patients have subtherapeutic trough concentrations and absent antidrug antibodies. This scenario is a consequence of rapid drug clearance, classically in the setting of a high inflammatory burden. Immune-mediated drug failure is seen in patients who have low or undetectable trough concentrations and high titers of antidrug antibodies. Published guidance has suggested minimal therapeutic target trough levels; infliximab >5 μg/mL, adalimumab >7.5 μg/mL, and certolizumab pegol >20 μg/mL (151,153). Of note, patients with a history of anti-TNF antibodies are at a greater risk of developing antidrug antibodies to the next agent within the same class. Therefore, combination therapy with immunomodulators such as the thiopurines or methotrexate should be considered (154).
There is a suggestion that higher anti-TNF drug levels are associated with better rates of fistula healing (155–161). This association has been found in numerous trials; however, the quality of many of these studies have been limited as a consequence of their use of subjective outcomes and observational designs. There are, however, no high quality, interventional data available.
We note that this is partly because performing high quality clinical trials in perianal fistulizing CD can be challenging and costly. Moreover, conducting, and interpreting therapeutic drug monitoring studies impose their own challenges. Drug level concentrations may vary between laboratories and assays, which limits the extrapolation and comparison of results. Moreover, endpoints may vary across studies and patient demographics and selection may also complicate the interpretation of the data. Ultimately, further interventional, randomized controlled trials looking into the relationship between drug exposure and fistula outcomes are needed.
Working definitions of disease activity and prognosis
Disease activity reflects the combination of symptoms and endoscopic findings, whereas prognosis is the compilation of factors predictive of a benign or a more complicated course with greater likelihood of surgery and/or disease-related disability.
An individual may be in clinical, endoscopic, histologic, or surgical remission. Although most clinical trials have used the CDAI to assess therapeutic outcomes, a more clinical working definition for CD activity is of greater value for the practicing provider to guide therapy in an appropriate manner. Of note, the CDAI is a measurement meant primarily for clinical trial use, not clinical practice. For clinical practice, disease activity is assessed by a combination of clinical symptoms (e.g., abdominal pain, stool frequency) plus elevated inflammatory biomarkers or disease activity identified on radiologic or endoscopic assessments. Clinical remission, corresponding to a CDAI score 145). Endoscopic remission is described as the absence of ulceration with minimal mucosal abnormalities on ileocolonoscopy. Histologic remission refers to the absence of inflammatory cells, particularly neutrophils, on mucosal biopsy (145). Surgical remission indicates patients who are status post surgery such as ileocolonic resection and have no residual active disease during postoperative endoscopic assessment. Individuals who require the use of conventional corticosteroids to achieve clinical well-being are said to be steroid-dependent and are not considered to be in remission because of adverse events which accrue in patients with chronic use of systemic corticosteroids.
Individuals with mild disease are at lower risk of disease progression or future surgery and have no systemic signs of toxicity such as fevers, unintentional weight loss, or inability to tolerate oral intake. Objectively, biomarkers (CRP, calprotectin) may be normal to slightly elevated, and there is only limited anatomic involvement with scattered aphthous erosions or few superficial ulcers. These individuals do not have severe endoscopic lesions, strictures, fistulizing, or perianal disease (15,162,163).
Individuals are considered to have moderate–severe disease if they have not responded to treatment for mild–moderate disease or if they present with more prominent symptoms such as fever, significant weight loss, abdominal pain or tenderness, intermittent nausea or vomiting. Inflammation-related biomarkers (e.g., CRP, albumin, calprotectin) are more likely to be abnormal, and other factors such as anemia or vitamin/mineral deficiencies may also be present. These patients typically have greater endoscopic disease burden including larger or deeper ulcers, strictures, or extensive areas of disease and/or evidence of stricturing, penetrating, or perianal disease.
Individuals with severe/fulminant disease have persistent symptoms despite the introduction of conventional corticosteroids and/or advanced therapies or present with high fevers, evidence of intestinal obstruction, significant peritoneal signs such as involuntary guarding or rebound tenderness, cachexia, or evidence of an abscess usually requiring hospitalization. They also have endoscopic or radiographic evidence of severe mucosal disease.
There has been a move by regulators to require patient-reported outcomes for regulatory approval of new therapeutic agents for the treatment of patients with CD. The primary endpoint is to measure an endpoint that matters to patients. The European Medicines Agency is moving away from the use of the CDAI to focus on patient-reported outcomes such as stool frequency and abdominal pain and separately, objective measures of disease, such as findings on endoscopy (164). The US Food and Drug Administration (FDA) had done the same initially but is currently back to using the CDAI and objective measures of disease such as findings on endoscopy as primary clinical trial endpoints (165).
Mucosal healing
Key concept
Mucosal healing has become an important target goal when assessing efficacy of treatment for IBD (145). In patients with CD, mucosal healing has traditionally been defined as the absence of ulceration visualized during endoscopy (166,167). There are a limited number of studies that have examined the long-term impact of endoscopic healing on the clinical course of disease. In patients with early-stage CD, complete endoscopic healing after 2 years of therapy predicts sustained steroid-free, clinical remission 3 and 4 years out from initiation of treatment (168). Other clinical outcomes associated with mucosal healing in CD include decreased rates of surgery and hospitalizations (169–171). With histologic remission, typically defined as absence of neutrophils on biopsy in addition to endoscopic healing, there may be lower associated risks of clinical relapse, corticosteroid use, or treatment escalation (172).
There are several scoring systems that assess ulcer size, depth, and distribution throughout the ileum and colon including the SES-CD, the Crohn’s Disease Endoscopic Index of Severity, Simplified Endoscopic Mucosal Assessment for Crohn’s Disease (simplified endoscopic mucosal assessment for CD, based on the SES-CD), and the Rutgeerts score to evaluate the postoperative neoterminal ileum (Supplementary Information online) (79,173–175). Allez et al described the severe endoscopic lesion group as patients with large confluent and deep ulcers that occupy >10% of the surface area of at least 1 segment of the colon. These lesions, particularly in the ileum and rectum, may be more refractory to medical therapy (176–178). The SES-CD has been helpful to translate endoscopic activity into clinically meaningful findings that are easier for the clinician to understand. SES-CD scores may be categorized as 0–2 representing endoscopic remission; mild (3–6), moderate (7–15), and severe (>15) disease activity. Converting these findings into descriptive terms, mild endoscopic activity would consist of limited aphthous erosions involving less than 10% of the surface area and/or altered vascular pattern, erythema, and edema affecting less than 50% of the surface area. Moderate endoscopic activity would consist of erosions or superficial ulcers taking up >10% but less than 30% of the surface area and severe disease as large ulcers >2 cm (79,179).
The SES-CD scoring system has been used prospectively to assess mucosal healing in patients treated with advanced therapies (i.e., anti-TNFs, anti-integrins, anti-ILs, Janus kinase [JAK] inhibitors) demonstrating that changes over time can be measured. Furthermore, there is a strong correlation between improvement in SES-CD and clinical outcomes of response and remission (79,180–184). For patients who have undergone ileocolonic resection, assessment of the small intestine just proximal to the anastomosis, recommended within the first year after surgery, may identify postoperative endoscopic recurrence well before the clinical recurrence of CD (185).
MEDICAL THERAPY
General approaches
Recommendation
Medical treatment of CD is usually categorized into induction and maintenance therapy. Regimens are generally chosen according to the patient’s risk profile and disease severity with a goal to achieve clinical and biomarker response within 12 weeks of treatment initiation followed by durable steroid-free control of disease activity including both clinical and endoscopic remission. It is important to acknowledge, however, CD clinical trials have only recently incorporated objective outcomes such as endoscopic improvement as a coprimary outcome (165). Another therapeutic goal is to prevent disease complications, such as strictures and fistulae. While medical therapy may be successful in some patients with fistulizing disease, including perianal disease, there is less evidence for medication efficacy in stricturing CD given the known fibrotic component of chronic strictures. Medical therapy used to treat CD primarily include supportive care including dietary-based strategies, corticosteroids and advanced therapies including anti-TNF agents, anti-integrins, anti-ILs, and JAK inhibitors.
A recent open-label randomized controlled trial (PROFILE—Predicting Outcomes for Crohn’s Disease Using a Molecular Biomarker) evaluated 2 separate approaches to the management of newly diagnosed CD, early combined immunosuppression with infliximab plus immunomodulator or accelerated step-up therapy where conventional management with corticosteroids was followed by immunomodulator, then followed by infliximab use. The primary endpoint was sustained steroid-free and surgery-free remission at week 48. Those in the early combined group were significantly more likely to achieve steroid-free and surgery-free remission (79% vs 15%). This study demonstrates the benefit of early intervention with advanced therapy as compared to a serial approach first requiring the use of conventional therapy (186).
Mild-to-moderate CD
Recommendations
Key concept
When treating patients with CD, therapeutics are chosen based on the patient’s clinical presentation and prognosis, that is, the risk of progression of their disease (see “Natural History” section). Risk factors for progression include young age at the time of diagnosis, ileal disease location, serological response to specific microbial antigens, initial extensive bowel involvement, presence of perianal/severe rectal disease, deep ulcers, and penetrating or stricturing phenotype at diagnosis (14,15,187). There is also a subgroup of patients who rapidly progress to complicated disease behaviors with stricturing disease leading to possible bowel obstruction, internal penetrating fistulas, or both, which are associated with greater likelihood of needing CD-related surgery (15).
Treating the patient with disease on the milder spectrum presents a conundrum. On the one hand, agents proven to be effective in patients with moderate-to-severe disease, such as the biologic agents, are undoubtedly effective in mild disease as well, even if such patients were not explicitly studied in randomized controlled trials. On the other hand, the risk of adverse effects and high cost of such agents may not be justifiable in a low-risk population. Unfortunately, few agents studied in milder disease populations have proven to be effective. The desire to avoid overtreating disease and exposing the mild patient to unnecessary risk has led to the widespread utilization of largely ineffective agents whose use cannot be justified by clinical evidence. For example, 5-aminosalicylates (5-ASAs) remain widely prescribed for the treatment of CD, despite considerable evidence demonstrating their lack of efficacy.
Patients deemed to be at low risk for progression of disease may be monitored with supportive care strategies directed at symptom control, but they must be followed carefully for signs of disease worsening or progression. Because the primary goal of CD treatment is normalization or at least substantial improvement of objective indicators of mucosal inflammation, providers should recognize that inadequate disease treatment based on expectant observation and alleviation of symptoms, especially for higher-risk patients, may expedite disease progression and development of complications.
Key concept
5-Aminosalicylates.
5-ASAs are topical anti-inflammatory agents which exert their effects within the lumen of the intestine. Although their use in UC has been well-established, the effectiveness of 5-ASAs in CD has not been supported by the published evidence. Oral mesalamine was not more effective compared with placebo for induction of remission and achieving mucosal healing in patients with active CD (188–191). Sulfasalazine, 3–6 g daily in divided doses, may be a modestly effective therapy for treatment of symptoms of patients with mild colonic CD and/or ileocolonic CD, but not isolated small bowel disease. However, sulfasalazine was not more effective than placebo for achieving mucosal healing in patients with CD even when used in combination with corticosteroids to induce then maintain remission. While 5-ASA suppositories or enemas are effective for induction and maintenance of remission for patients with mild to moderate UC; the role of topical mesalamine in CD, although commonly used, is of limited benefit (188,192–194).
5-ASAs have also been extensively studied for maintenance of medically induced remission of CD with equivocal benefit. There were 11 placebo-controlled trials of 5-ASAs, with doses ranging between 1 and 4 g per day and maintenance treatment duration between 4 and 36 months. Four of the studies reported a significant decrease in CD relapse compared with placebo; however, the other 7 studies showed no prevention of relapse (195–205). There were 5 meta-analyses evaluating the efficacy of mesalamine for the maintenance of medically induced remission in patients with CD. The therapeutic advantage between mesalamine and control was 206–209). Given the totality of data, 5-ASAs are not recommended for maintenance of medically induced remission of CD.
Budesonide.
Corticosteroids are primarily used to reduce the signs and symptoms of active luminal CD and to potentially induce clinical remission; however, corticosteroids have not been consistently effective in achieving mucosal healing for patients. Ileal-release steroid formulations may be used for mild to moderate disease, whereas systemic corticosteroids are used for moderate to severe disease. They have historically been used as a bridge to permit symptom control until immunomodulators and/or biologic agents become effective and enable mucosal healing.
Although not as effective as conventional oral corticosteroids such as prednisone, controlled-ileal release (CIR) budesonide may be effective for short-term relief of symptomatic mild-to-moderate CD in patients with disease confined to the terminal ileum and right colon (210). CIR budesonide is a pH-dependent ileal release oral corticosteroid formulation with high topical activity and low systemic bioavailability (∼10%–20%). CIR budesonide has been demonstrated to be effective in randomized placebo-controlled trials for induction of remission in active mild-to-moderate ileocecal CD (210–212). The lesser efficacy of CIR budesonide is balanced against the agent’s release profile, limited to the ileum and right colon, and its topical activity with extensive first-pass hepatic metabolism, minimizing systemic exposure to corticosteroid effects.
Budesonide should not be used to maintain remission in mild to moderate CD. There were 6 randomized placebo-controlled studies evaluating maintenance of remission with budesonide. The 12-month relapse rates for 3–6 mg budesonide daily ranged from 40% to 74% and were not significantly different than placebo (213–217). Four meta-analyses have been published on the efficacy of budesonide dosed at 3–6 mg daily for maintenance of remission in CD. The results are mixed with most showing no benefit in maintenance of remission or in achieving mucosal healing, with only a slight improvement in mean time to symptom relapse but increased adverse events compared with placebo (218–221). In a Cochrane Database review of 12 studies (total 1,273 patients) which included 8 studies that compared budesonide with placebo, 1 study comparing budesonide with 5-ASAs, 1 compared with corticosteroids, 1 compared with azathioprine, and 1 comparing 2 doses of budesonide, budesonide was not effective for maintenance of remission beyond 3 months after induction. Although budesonide did seem to have some benefit in symptom response and a longer time for disease relapse, the risks for treatment-related adverse events including higher rates for adrenocorticoid suppression was observed (222). Therefore, whether disease activity recurs after a course of budesonide or whether there is an incomplete response to budesonide, additional evaluation is necessary to determine whether an advanced therapy is warranted vs whether other diagnoses are present that may be contributing to symptom presentation.
Key concept
Antimicrobial therapy.
In patients with CD, it is hypothesized that the development of chronic intestinal inflammation is caused by an abnormal immune response to normal flora in genetically susceptible hosts. The involvement of bacteria in CD inflammation has provided the rationale for including antibiotics in the therapeutic armamentarium. The precise mechanisms whereby broad-spectrum antibiotics are beneficial in the treatment of a subset of patients with CD are uncertain. Several proposed mechanisms of efficacy include direct immunosuppression (e.g., metronidazole), elimination of bacterial overgrowth, and abolition of a bacterially mediated antigenic trigger.
Although widely used in the past, the primary role of antibiotics for the treatment of luminal CD has not been supported by the evidence (223,224). Metronidazole is not more effective than placebo at inducing remission in patients with CD (225,226). Ciprofloxacin has shown similar efficacy to mesalamine in active CD but has not been shown to be more effective than placebo to induce remission in luminal CD. Neither of these agents has been shown to heal the mucosa in patients with active luminal CD (226–229). Rifaximin, a nonabsorbable predominantly luminally active antibiotic, has been studied for the induction of remission with a potential efficacy signal at higher than conventional dosing. However, the cumulative evidence has yielded inconsistent results, and maintenance of remission data is lacking (230,231).
Antibiotics may be used an adjunctive treatment for patients with CD with complications of CD. For example, for patients with perianal CD, the addition of antibiotics in combination with biologics or thiopurines to improve outcomes such as fistula closure may be considered (232,233). Antibiotics such as the nitroimidazoles may also have a role for postoperative prophylaxis for patients with low risk of CD recurrence postresection (234). Broad-spectrum antibiotics are used for the treatment of pyogenic complications (e.g., intra-abdominal, mesenteric, or perianal abscesses) in patients with CD.
The relationship of mycobacterial disease, specifically Mycobacterium avium paratuberculosis (MAP), to the development of CD has been extensively evaluated. The absence of MAP in all tissue examined (even when assessed by PCR) and the lack of significant patient disease benefit when treated with multidrug regimens has led to the recommendation that anti-MAP therapy should not be used to treat patients with active CD. Anti-MAP therapy has not been shown to be effective for induction or maintenance of remission or mucosal healing in patients with CD (235,236).
Key concept
Diet.
Some studies suggest that dietary therapies, including elemental, semielemental, and defined diets, may be effective for select patients with CD to reduce clinical symptoms and disease activity scores. When discussing dietary-based strategies as primary treatment, the CD patient’s current disease activity and risks for disease progression and complications need to be considered. Most of the diet-based studies were performed primarily among pediatric patients with CD. In the adult patient population, these benefits are often not durable with symptoms and active inflammation reoccurring on resumption of an unrestricted diet (237,238). The CD exclusion diet has been developed to reduce exposure to potential proinflammatory elements and has been shown in several studies to induce remission primarily among patients with mild to moderate CD (237,239,240). The DINE-CD study, a randomized trial comparing the specific carbohydrate diet to the Mediterranean diet for adult patients with CD, revealed no differences in symptomatic remission and biomarker response between the 2 diet-based strategies for patients with mild-to-moderate CD (241). Adherence to a Mediterranean diet for at least 6 months was associated with improvement in biomarkers and quality of life among patients with CD (242), Therefore, a primary dietary-based treatment approach should be limited to patients with limited disease and low risks of disease progression. Routine monitoring with symptom, laboratory, and diagnostic assessments remains important to identify disease progression (243).
Moderate-to-severe CD
Corticosteroids recommendations
Patients experiencing moderate-to-severe symptoms or who have multiple high-risk features for disease progression and complications require treatment with advanced therapy. Conventional corticosteroid treatment, such as prednisone and methylprednisolone given orally or intravenously for more severe disease, is effective in alleviating signs and symptoms of a flare (244). The appropriate prednisone equivalent doses used to treat patients with active CD range from 40 to 60 mg/d (245,246). These doses are typically maintained for 1–2 weeks and tapered at 5 mg weekly until 20 mg and then 2.5–5.0 mg weekly. Corticosteroid tapers should generally not exceed 3 months. Oral prednisone doses or equivalent doses in other oral steroids exceeding 60 mg a day are not recommended. There have been no adequately powered comparative trials between different steroid-tapering regimens in the treatment of patients with CD.
The use of corticosteroids should not exceed 3 continuous months without attempting to introduce corticosteroid-sparing agents (such as biologic therapy or immunomodulators). Even short-term use may be accompanied by important adverse events, such as severe infections, accelerated bone loss, elevated blood glucose, glaucoma, weight gain, venous thromboembolic events (5-fold increased risk), and cardiovascular disease (38,244,247,248).
Despite their effectiveness in reducing signs and symptoms of active CD, nearly 1 in 4 patients will have prolonged exposure to corticosteroids (i.e., greater than 6 months), particularly earlier in their disease course with approximately 15% of patients becoming steroid-dependent with an inability to taper without subsequent recrudescence of symptoms (249). In a meta-analysis including 403 patients with surgically or medically induced remission, corticosteroids were not effective at maintaining remission (250). The rates of remission were no different between placebo and corticosteroids at 6, 12, and 24 months. Prolonged or recurrent corticosteroid use may decrease the effectiveness of steroid-sparing agents for mucosal healing, even among those who experience symptomatic relief. In addition, corticosteroids are implicated in the development of perforating complications (abscess and fistula) and are relatively contraindicated in those patients with such manifestations. For all these reasons, corticosteroids should be used sparingly in CD. Once started, care should be taken to ensure that corticosteroids are successfully discontinued with a gradual taper and steroid-sparing agents added.
Immunomodulators recommendations
Key concept
Because of their relatively slow onset of action of 8–12 weeks, thiopurines are not effective agents for induction of remission among patients with active, symptomatic disease (251,252). There are 3 scenarios by which a thiopurine is used after corticosteroid induction of remission. One scenario is to initiate the thiopurine at the time of the first course of corticosteroid, the second is after repeated courses of corticosteroids or in patients who are corticosteroid-dependent (i.e., unable to taper the steroid without CD relapse), and the third is as a concomitant medication with an anti-TNF agent to reduce the risk of development of antibodies and improve pharmacokinetic parameters. For patients with moderate-to-severe CD who remain symptomatic despite current or prior corticosteroid therapy, the thiopurine analogs azathioprine (at maximal doses of 1.5–2.5 mg/kg/d) or 6-mercaptopurine (at maximal doses of 1–1.5 mg/kg day) may be used as a steroid-sparing maintenance agent. TPMT testing should be checked before initial use of azathioprine or 6-mercaptopurine to identify patients at increased risk for thiopurine-associated myelosuppression (253,254). Variants in the NUDT15 gene have also been demonstrated to affect thiopurine metabolism leading to increased medication related toxicity among particularly among people of East-Asian, Latino, and Native American ancestries. Testing for NUDT15 genetic variants should be considered if available (255–257).
For newly diagnosed pediatric CD, 6-mercaptopurine, dosed at 1.5 mg/kg/d, administered in combination with the first course of corticosteroids, has demonstrated efficacy (258). Presumably, the same efficacy would be realized with azathioprine in an adult population, but a randomized open-label study of early use of azathioprine in CD was unable to demonstrate a benefit with respect to time in clinical remission (259).
Adverse effects of azathioprine and 6-mercaptopurine include allergic reactions, pancreatitis, myelosuppression, nausea, infections, hepatotoxicity, nonmelanoma skin cancer, and lymphoproliferative disorders (260,261). The risks of skin cancer and lymphoma have been demonstrated in multiple observational studies with increasing risks attributed to ongoing use of thiopurines, duration of exposure, and increasing age. While, reassuringly, these risks seems to decrease with medication discontinuation, the aggregate risks of adverse effects with longer-term use of these agents needs to be factored along with the efficacy data from earlier clinical trials and the availability of other CD options to treat moderate-to-severe disease (262–265). Although these agents may be considered as steroid-sparing maintenance agents, accumulating risks associated with thiopurine exposures may outweigh the original steroid-sparing benefit particularly with the other mechanisms of action now available, which have a more favorable safety profile.
Methotrexate is also effective as a corticosteroid-sparing agent for the maintenance of CD remission (266–268). Parenterally (subcutaneous or intramuscular) administered methotrexate at doses of 25 mg per week is effective for maintenance of remission in CD after steroid induction (268,269). If steroid-free remission is maintained with parenteral methotrexate at 25 mg per week for 4 months, the dose of methotrexate may be lowered to 15 mg per week (270). Patients with normal small bowel absorption may be started on or switched from parenteral to oral methotrexate at 15–25 mg once per week; however, controlled data with oral methotrexate as a primary treatment for CD are lacking. For patients with extensive small bowel disease or risk factors for malabsorption, the bioavailability of oral methotrexate at higher dosages may be variable. Thus, parenteral methotrexate may be the preferred route of administration in this context (271).
Adverse effects related to methotrexate include nausea and vomiting, hepatotoxicity, pulmonary toxicity, bone marrow suppression and skin cancer, and likely lymphoma; however, an escalated risk of lymphoma has not been conclusively demonstrated in patients with CD. The white blood cell counts and liver chemistries should be routinely monitored during their use. When prescribed to women with child-bearing capability, methotrexate should be administered only if highly effective contraception is in place (272,273).
Thiopurines or methotrexate may also be used as adjunctive therapy for reducing immunogenicity for patients on anti-TNF therapy (6-mercaptupurine or azathioprine typically at reduced doses and methotrexate 12.5–15 mg orally once weekly) (65,274,275). Antidrug antibodies associated with anti-TNF therapies, particularly infliximab and adalimumab, can develop as early as the first 100 days of treatment, particularly with anti-TNF monotherapy. Factors such as active smoking status, increased body mass index, and anti-TNF monotherapy may be associated with lower drug levels at week 14 and greater risks of loss of response, whereas earlier initiation of combination therapy with immunomodulators may yield more durable effectiveness (65,276).
Anti-TNF agents recommendations
The anti-TNF-α therapies approved for moderate to severe CD include infliximab, a chimeric mouse-human IgG1 monoclonal antibody available as intravenous infusions and subcutaneous injections; adalimumab, a subcutaneous fully humanized IgG1 monoclonal antibody; and certolizumab pegol, a subcutaneous pegylated Fab fragment to TNF-α. These biologic agents are effective for treating patients with CD with objective evidence of active disease and inadequate response to corticosteroids, thiopurines, and/or methotrexate, especially patients with multiple risk factors for disease progression (i.e., younger age at diagnosis, ileal disease location, extensive disease, larger/deep ulcers on endoscopy). The anti-TNF agents have a potentially rapid onset of action occurring as early as within the first 2 weeks of treatment initiation (277). However, treatment with anti-TNF agents also seems to be more effective when given earlier in the course of disease because rates of response and remission are higher if given within 2 years of onset of disease. In the PROFILE study, top-down treatment with combination infliximab plus immunomodulator achieved substantially better outcomes at 1 year than accelerated step-up treatment. The use of biomarkers did not show clinical utility. Therefore, top-down treatment should be considered the standard of care for patients with newly diagnosed active CD (186).
Infliximab, adalimumab, and certolizumab pegol are also effective for maintenance of medically induced remission in luminal CD, and numerous clinical trials have supported the use of anti-TNF agents beyond induction (278–284). In a meta-analysis including 14 clinical trials (total of 3,995 patients), infliximab, adalimumab, and certolizumab were effective for maintenance of remission at weeks 20–30 among patients with CD who responded to induction therapy (285). In another meta-analysis of 5 trials (total of 1,390 patients), the RR of relapse at weeks 26–56 among patients treated with an anti-TNF agent compared with placebo was 0.71 (95% CI 0.65–0.76). The number needed to treat with an anti-TNF agent to prevent 1 patient with CD to relapse after remission of active disease achieved was 4 (95% CI 3–5) (286). In a Cochrane Database review, the pooled analysis of 5 or 10 mg/kg infliximab every 8 weeks was found to be superior to placebo for maintenance of remission and clinical response at week 54; 400 mg certolizumab pegol every 4 weeks was superior to placebo for maintenance of remission and clinical response at week 26, and 40 mg adalimumab every other week or every week was superior to placebo for maintenance of clinical remission at week 54 (287).
Infliximab is the only anti-TNF agent available as either an intravenous or subcutaneous maintenance therapy for patients with CD. The initial phase 1 study highlighted the pharmacokinetic noninferiority of subcutaneous vs intravenous infliximab in terms of efficacy, safety, and immunogenicity (288). In the phase 3 randomized controlled trial, the LIBERTY trial, comparing maintenance dosing of subcutaneous infliximab with placebo after standard infliximab induction, 62% of subcutaneous infliximab-treated patients achieved clinical remission at week 54 compared with 32% of placebo patients. Over 50% of subcutaneous infliximab patients had endoscopic response compared with only 18% of placebo-treated patients (289). Subsequently, additional studies of subcutaneous infliximab yielded similar findings, highlighting the effectiveness and safety of this agent as another option for patients where infliximab maintenance is recommended (290). However, some patients transitioning to subcutaneous infliximab 120 mg every other week may require dose escalation to 240 mg subcutaneous every other week to achieve or recapture response. The REMSWITCH study was a multicenter observational study which evaluated patients in steroid-free clinical remission on variable but stable dosing of infliximab (5 or 10 mg/kg every 4, 6, or 8 weeks) transitioning to subcutaneous infliximab 120 mg every 2 weeks. Disease relapse was more likely to occur among patients taking higher or more frequent dosing of infliximab by weeks 16–24 postswitch: 10.2% (5 mg/kg every 8 weeks), 7.3% (10 mg/kg every 8 weeks), 16.7% (10 mg/kg every 6 weeks), and 66.7% (10 mg/kg every 4 weeks). Importantly, dose escalation to 240 mg every other week led to recapture clinical remission in 93.3% and clinical + biomarker remission (based on FC) in 80% of patients. Patients who were receiving infliximab 10 mg/kg every 4 weeks and had an FC >250 μg/g were more likely to experience a flare, suggesting these patients may need a dose of infliximab 240 mg subcutaneous every 2 weeks at initiation (291).
Combination therapy with an anti-TNF agent and an immunomodulator has been demonstrated to improve short-term efficacy compared with monotherapy (292–294). Patients with CD treated with infliximab plus azathioprine or infliximab monotherapy were more likely to achieve corticosteroid-free clinical remission than patients receiving monotherapy azathioprine and with no notable differences in safety in the SONIC trial (292). The addition of a thiopurine or methotrexate with anti-TNF therapy may also improve pharmacokinetic parameters and reduce immunogenicity (293,294). In a recent genomic subanalysis of a prospective observational study of patients with CD starting adalimumab or infliximab, carriers of HLA-DQA1*05 were at an increased risk for development of antibodies against infliximab and adalimumab (65). However, earlier initiation of combination therapy may protective against immunogenicity allowing for greater persistence of treatment (276). Therefore, combination therapy may be the preferred strategy of treatment for patients with higher-risk CD who do not have risk factors precluding its use.
A 2023 meta-analysis of 13 studies found that HLA-DQA1*05 variants are associated with a higher risk of immunogenicity and secondary loss of response in patients with immune-mediated inflammatory diseases treated with TNF-α antagonists. The risk of immunogenicity in those patients with HLA-DQA1*05 variants was 75% higher than noncarriers, and the risk of secondary loss of response was 123% higher than noncarriers with a positive predictive power of 30% and a negative predictive power of 80%. The meta-analysis also found that proactive therapeutic drug monitoring can modify the association between HLA-DQA1*05 variants and immunogenicity (295).
The benefits and risks of combination therapy must be individualized. There is a higher risk of lymphoma in patients treated with azathioprine or 6 mercaptopurine, especially among older patients and with longer duration of exposure (251). There is also a rare but increased risk of hepatosplenic T-cell lymphoma particularly for younger males treated with combination anti-TNF and thiopurine therapy (296). For patients where combination therapy is considered higher risk, optimized infliximab monotherapy with targeted therapeutic drug monitoring may be considered, avoiding long-term use of thiopurines and potential associated toxicities (297,298). Some evidence suggests that immunogenicity may be prevented with proactive therapeutic drug monitoring and maintaining robust trough levels of the TNF antagonist while on infliximab monotherapy because the primary effect of immunomodulator in combination therapy is in nonspecifically increasing drug trough concentrations (299). In a post hoc analysis, among patients with CD with similar infliximab serum concentrations, combination therapy with azathioprine was not more effective than infliximab monotherapy (300). However, a meta-analysis of randomized controlled trials comparing proactive therapeutic drug monitoring to conventional approaches did not identify a clinical benefit for anti-TNF treated patients (301).
The safety profile of anti-TNF agents is generally favorable, but a small percentage of patients may experience severe side effects. A meta-analysis of 21 anti-TNF clinical trials including 5,356 patients with CD concluded that anti-TNF therapy did not increase the risk of serious infection, malignancy, or death compared with placebo (285). However, clinical trials of 1 year duration may not be sufficiently large or long enough to detect adverse events. In addition, these agents are safe to use during preconception planning, throughout pregnancy and postpartum (302,303). Individuals at increased risk for use of anti-TNF therapy include patients with prior demyelinating disorders (e.g., optic neuritis and multiple sclerosis), congestive heart failure, and individuals with a history of lymphoma or malignancies. Infectious complications may occur with the use of these agents, and thus, vigilance is advocated when treating these patients including routine laboratory monitoring, counseling regarding potential adverse effects, and recommended pretreatment assessments (304).
Before anti-TNF therapy is considered for use in patients with CD, pretreatment screening for infections and laboratory abnormalities is required. Testing for latent and active tuberculosis should be undertaken as well as assessment of patient risk factors for exposure. Interferon-γ release assays are likely to complement the tuberculin skin test and are preferred in patients who are Bacillus Calmette-Guerin vaccinated, if available. Similar testing and therapy should also be considered before corticosteroids or other immunomodulators in patients at high risk of tuberculosis. If latent tuberculosis is detected, initiation of chemoprophylaxis with antituberculous therapy should be initiated for several weeks before administration of anti-TNF therapy. It may be appropriate to consider a second tuberculin skin test in an immunocompromised host after the initial test is negative. This is classically done 1–3 weeks later (305).
Before initiation of most advanced CD therapies, patients should be screened for hepatitis B virus (HBV) using a panel including hepatitis B surface antigen (HBsAg), hepatitis B core antibody (anti-HBc), and hepatitis B surface antibody (anti-HBs) because immunosuppressive medications can lead to HBV reactivation. If a patient is seronegative for hepatitis B, vaccination (using a recombinant vaccine) should be initiated, ideally before the introduction of biologic therapy. Assessment of serologic response is advocated after vaccination. If a patient is positive for HBsAg, antiviral prophylaxis should be initiated before starting the biologic therapy. Before and during treatment with biologic and/or immunomodulator therapy, patients who are HBsAg (hepatitis B surface antigen)-positive carriers should receive treatment with antiviral agents (nucleoside/nucleotide analogs) to avoid hepatitis B flare and liver failure. Those who are actively infected should defer acute biologic therapy initiation until adequate duration of Hepatitis B antiviral therapy has been initiated. Patients who are anti-HBc positive, HBsAg negative require further evaluation with HBV DNA testing to assess for potential reactivation risk. In a relatively recent meta-analysis, the risk of HBV reactivation in anti-HBc-positive patients with non-hematological diseases was 3.6% (306). Quantification of anti-HBc antibodies can help distinguish occult hepatitis B infection from a past HBV infection (307). Detection of anti-HBc antibodies serves as a surrogate marker of occult HBV infection. Occult HBV infection has been defined as the detection of HBV DNA in the liver tissue (gold standard) or in the blood (308). Considering this, all patients who are HBCore antibody–positive should have HBV DNA assessed at the time of diagnosis of HBCore positivity and periodically thereafter in addition to undergoing routine liver chemistry assessments.
Other appropriate vaccinations (pneumococcal vaccine, varicella, human papilloma virus, inactivated influenza vaccine, hepatitis A vaccine, severe acute respiratory syndrome coronavirus 2, and herpes zoster) should be initiated ideally before use of biologic therapy. The use of live attenuated vaccines should be avoided in patients with IBD using immunomodulator therapy or biologic therapy (e.g., measles–mumps–rubella, vaccinia, yellow fever, live attenuated nasal influenza vaccine, varicella, oral polio, and Bacillus Calmette-Guerin). Vaccination status ideally should be reviewed and updated at diagnosis. Live vaccines should be avoided after initiation of systemic immune suppressive therapy (136).
Biosimilars
Key concepts
There are currently multiple biosimilars for infliximab, adalimumab, and ustekinumab that have regulatory approval for use in patients with moderate-to-severe CD. Unlike the generics of small-molecule drugs, exact replicas cannot be made of biologics because of their structural complexity and complicated manufacturing process. A biosimilar is a biological product that is highly similar to the reference product notwithstanding minor differences in clinically inactive components; there are no clinically meaningful differences between the biosimilar product and the reference product regarding safety, purity, and potency (309). The biosimilar must have the same strength and dosage form (injectable, for example) and route of administration as the reference product. The approval pathway for biosimilars differs from that of the originator biologic—the primary emphasis is on analytical characterization, preclinical/animal studies, and pharmacokinetic studies. Once these have been demonstrated, clinical studies demonstrating pharmacokinetics, efficacy, and safety that are similar to the originator biologic in 1 indication for which the drug is approved are often sufficient for extrapolation to all disease indications. Interchangeable biosimilars represent agents that are similar to the licensed reference product that are expected to produce the same clinical result to the reference product in any given patient, even after multiple switches between the reference and biosimilar products. An interchangeable biosimilar can be substituted at the pharmacy level without the intervention of a health care provider. The ability of a pharmacist to substitute a biosimilar for an originator drug will be determined by each state’s pharmacy board, not by the FDA interchangeability designation (309–311).
The potential advantages of biosimilars include cost savings and improved patient access to advanced therapies earlier in the disease course. In a physician survey, more than 50% of providers commented that cost was a factor when recommending treatment options for patients. For most respondents, with the use of biosimilars, there was at least a 30% reduction in cost without affecting shared therapeutic decision-making (312). There exist concerns by some that small differences in the efficacy and safety of these molecules may be magnified in less anti–TNF-responsive diseases such as IBD, leading to altered immunogenicity and drug metabolism. However, the overwhelming data evaluating biosimilars for moderate-to-severe CD indicate no differences regarding efficacy, safety, and treatment persistence (313). A large randomized, non-inferiority phase 4 trial (NOR-SWITCH) of patients with CD, UC, spondyloarthritis, rheumatoid arthritis, psoriatic arthritis, and plaque psoriasis showed that switching from infliximab originator to CT-P13 (biosimilar) was not inferior to continued therapy with the originator (314). In the NOR-SWITCH open label extension study, patients continuing with the originator infliximab through week 52 were transitioned to the biosimilar with no noted differences in efficacy or safety through week 78 compared with patients who continued on biosimilar infliximab (315). Pharmacokinetic profiles and immunogenicity rates were similar among patients switching to biosimilar infliximab compared with patients continuing with reference infliximab (316,317). Similarly, randomized controlled trial results from the VOLTAIRE-CD study demonstrated similar effectiveness, safety, and pharmacokinetic profiles when patients were switched from reference adalimumab to biosimilar adalimumab-adbm (318).
There are multiple ustekinumab biosimilars approved by the Food and Drug Administration for use in moderate-to-severe CD based on extrapolation from clinical trials in dermatology which determined similar efficacy, safety, and immunogenicity between reference and biosimilar agents (319–322). Two of the ustekinumab biosimilars have an interchangeable status (ustekinumab-auub, ustekinumab-ttwe). However, the regulatory process regarding interchangeability status for biosimilars is evolving as accumulating evidence consistently demonstrates equivalent clinical and safety outcomes across approved disease states (323).
Agents targeting leukocyte trafficking
Recommendation
Inhibitors of leukocyte trafficking recently have expanded the therapeutic options for patients with CD. Natalizumab, an anti-α4 integrin antibody, broadly interferes with leukocyte trafficking systemically and inhibits binding to both vascular cell adhesion molecule-1 and mucosal addressin cell adhesion molecule-1. Although effective in patients who have failed other agents, the risk of progressive multifocal leukoencephalopathy (PML), caused by John Cunningham (JC) virus, is as high as 1 in 100 among patients with JC virus antibody positivity, prior use of immunosuppressive agents, and 2 or more years of use. Treatment with natalizumab is best limited to those patients who are not seropositive for anti-JC virus antibody that should be checked before initiating therapy and at minimum every 6 months thereafter (324,325). With the availability of multiple newer agents with more favorable safety profiles, the other advanced therapies approved for moderate to severe CD should be used in lieu of natalizumab.
By contrast, vedolizumab selectively inhibits α4β7 integrin interaction with mucosal addressin cell adhesion molecule-1, making it relatively specific for leukocyte trafficking to the gut. Vedolizumab is used in adult patients with moderately to severely active CD who have had an inadequate response with, lost response to, or were intolerant to a TNF blocker or immunomodulator, or had an inadequate response with, were intolerant to, or demonstrated dependence on corticosteroid to achieve clinical response, clinical remission, corticosteroid-free remission, and mucosal healing (326–329).
In the GEMINI 2 study, vedolizumab 300 mg every 8 weeks was superior to placebo in maintaining clinical response and remission and achieved higher rates of corticosteroid-free remission at week 52 (329). In the GEMINI long-term safety (LTS) study which included patients who completed GEMINI 2, clinical remission was achieved in 74% after 152 weeks, including 82% among TNF antagonist naïve and 66% with prior TNF antagonist failure (330). Vedolizumab, given its favorable safety profile and potentially more gut-selective mechanism of action, can also be positioned before use of anti-TNF agents in the appropriate clinical context because nonresponse or intolerance to anti-TNF therapies is not a prerequisite for use. The onset of the clinical effect of vedolizumab may be slower compared with anti-TNF agents in patients with CD. Patients who have received prior treatment with anti-TNF agents require longer treatment, with efficacy rates at 10 weeks equaling those of anti–TNF-naive patients at 6 weeks (328). As a primary treatment for CD, vedolizumab may also be used as a monotherapy. Accumulating data suggest that the addition of concomitant immunomodulators such as methotrexate or thiopurines does not yield significant benefit in clinical, endoscopic, or pharmacokinetic outcomes (331,332).
A subcutaneous formulation of vedolizumab is also available with demonstrated efficacy for maintenance of remission for patients with CD. The VISIBLE 2 study was an open-label induction study where patients received vedolizumab intravenous 300 mg at week 0 and week 2 was followed by a maintenance dose (randomization of week 6 responders) of vedolizumab 108 mg subcutaneously or placebo every 2 weeks until week 52. More patients treated with subcutaneous vedolizumab maintenance therapy achieved the primary endpoint of clinical remission compared with placebo with similar adverse effect profiles (333). The results of the study demonstrated that exposure−efficacy relationships for intravenous and subcutaneous vedolizumab administration were comparable, confirming that both are equally effective during maintenance treatment (334).
Owing to the gut-selective nature of vedolizumab, there is no impact on the blood-brain barrier; therefore, vedolizumab has a more favorable safety profile compared with natalizumab. In the GEMINI LTS study of a total of 2,243 patients enrolled (1349 CD), vedolizumab discontinuation due to adverse events occurred in 229 (17%) of the patients with CD. The most common adverse events which led to treatment discontinuation was CD exacerbation (8%), and all other adverse events that led to discontinuation were reported in less than 1% of patients and included nasopharyngitis and arthralgia. There were no new trends for infection, malignancies, or infusion-related reactions, and no cases of PML were identified with 7,999 person-years of vedolizumab exposure in the GEMINI LTS study (335). There has been 1 single case of PML confirmed in over 470,000 person years of postmarketing vedolizumab exposure, in a patient with multiple contributing factors including a new diagnosis of HIV infection, low CD4 count, and concomitant prolonged immunosuppression. The Independent PML Adjudication Committee concluded that the most likely cause of PML in this patient was related to these factors and not vedolizumab-associated (336).
Agents targeting IL-12/23 (anti-p40 antibody) and IL-23 (anti-p19 antibody)
Recommendation
Key concept
Ustekinumab, an IgG1 anti-p40 antibody that inhibits IL-12 and IL-23, is effective for patients with CD with prior exposure to conventional therapies (e.g., corticosteroids, immunomodulators) and/or anti-TNF agents for induction and maintenance of remission (337). Subcutaneous ustekinumab monotherapy is effective for maintaining clinical remission among patients with moderate-to-severe CD who had demonstrated clinical response to an intravenous induction dose of ustekinumab, including patients who have not responded to corticosteroids, immunomodulators, and/or anti-TNFs, and this held true for those who had failed conventional therapy and those who had previously failed anti-TNF therapy (337). In the CERTIFI phase 2 trial, clinical remission at week 22 was greater among anti–TNF-resistant patients treated with ustekinumab compared with placebo (41.7% vs 27.4%, P = 0.03) (338). Among patients receiving maintenance doses of ustekinumab every 8 or 12 weeks in the phase 3 IM-UNITI trial, clinical remission was achieved in 53.1% and 48.8%, respectively, compared with 35.9% in the placebo group at week 44 (P = 0.005 and P = 0.04, respectively) (337,338). Data accrued through 5 years from IM-UNITI and long-term extension (LTE) using an intent-to-treat analysis of all patients randomized to ustekinumab at maintenance baseline found that 34.4% and 28.7% of patients in the every 8-week and 12-week groups, respectively, were in clinical remission at week 252, and the remission rates among the patients who entered in the LTE were 54.9% and 45.2%, respectively. Remission rates after 5 years for TNF antagonist-naïve patients was 44.2% and 21.4% for the TNF antagonist failure patient group treated with ustekinumab every 8 weeks (339). In the pivotal comparative effectiveness study of early bio-naïve with patients CD (SEAVUE—Safety and Efficacy of Adalimumab vs Ustekinumab for One Year) treated with standard dosing of ustekinumab and adalimumab as monotherapy, both agents were highly effective in achieving the primary endpoint of clinical remission at week 52 with no significant differences observed between the treatment arms (340). Both endoscopic and clinical remission endpoints have also been associated with trough concentrations of ustekinumab in CD based on analyses from the Phase 3 studies (UNITI-1, UNITI-2, IM-UNITI), while concentrations of ustekinumab were not affected by concomitant immunomodulators (341). The occurrence of antidrug antibodies to ustekinumab is also low and reported in 5.8% of patients in the LTE (339).
The overall adverse events, infections, and serious infection rates were similar in the combined ustekinumab and placebo groups through 5 years of IM-UNITI and LTE. Specifically, there was no evidence of increased risk for opportunistic infections or tuberculosis, malignancy, anaphylactic, and delayed hypersensitivity or death (339). An extensive safety database in patients with psoriasis demonstrated an excellent safety profile, without apparent increase in serious infections or malignancies (342). This favorable safety profile seems consistent with data from clinical trials of ustekinumab in CD, although with less accumulated long-term exposure, and despite higher doses being used. In a multicenter cohort of over 1,000 ustekinumab-treated patients with CD, rate of serious infections was only 3.4% and other noninfectious adverse events occurred in only 2.4% of patients (343).
Ustekinumab may be administered as monotherapy, although risks and benefits of combination therapy should be evaluated for each individual patient. In addition, dose optimization of ustekinumab may be a consideration for some patients with CD with inadequate response or loss of response to standard dosing. Approximately 20% of ustekinumab treated patients experience loss of response to treatment, and dose optimization can regain response in over 50% of patients, allowing for continuation of treatment without changing to a new mechanism of action (344). Results from a systematic review and meta-analysis reported 55% of patients with CD were able to achieve clinical response, 61% endoscopic improvement, and 29% mucosal healing following ustekinumab dose optimization (345).
Recommendation
Risankizumab, an IgG1 anti-p19 antibody that inhibits IL-23, is efficacious in patients with CD whose prior treatments have included corticosteroids, immunomodulators, or anti-TNF agents. There were 2 large induction studies (ADVANCE, MOTIVATE) where subjects received the risankizumab intravenous induction regimen (600 or 1,200 mg) at Weeks 0, 4, and 8 (346). Efficacy assessment was performed at week 12. All coprimary endpoints at week 12 were achieved with both doses of risankizumab (P ≤ 0.0001). In the ADVANCE trial, the clinical remission rate (CDAI ≤150) was 45% with risankizumab 600 mg and 42% with risankizumab 1,200 mg vs 25% with placebo. In addition, patient-reported outcomes improved with stool frequency and abdominal pain achieving clinical remission in 43% with risankizumab 600 mg and 41% with risankizumab 1,200 mg vs 22% with placebo. The endoscopic response rate was high at 40% with risankizumab 600 mg and 32% with risankizumab 1,200 mg vs 12% with placebo (346).
In the MOTIVATE study, where all patients with CD had intolerance or inadequate response to at least 1 biologic, the rate of clinical remission rate (CDAI ≤150) was 42% with risankizumab 600 mg and 40% with risankizumab 1,200 mg vs 20% with placebo. The rate of clinical remission as determined by patient-reported outcomes with stool frequency and abdominal pain was 35% with risankizumab 600 mg and 40% with risankizumab 1,200 mg vs 19% with placebo. The endoscopic response rate was 29% with risankizumab 600 mg and 34% with risankizumab 1,200 mg vs 11% with placebo (346).
The overall incidence of treatment-emergent adverse events was similar among the treatment groups in both trials. Specifically, there was no evidence of increased risk for opportunistic infections or tuberculosis, malignancy, anaphylactic, and delayed hypersensitivity or death. The rate of serious infections (1% in each trial with active therapy and 2% [MOTIVATE trial] and 4% [ADVANCE trial] with placebo), active tuberculosis (1 patient in placebo and 1 patient with active therapy) in the ADVANCE trial and none in the MOTIVATE trial, and adjudicated major adverse cardiovascular events (MACEs) were none in active therapy or placebo in either trial. This safety profile is consistent with prior risankizumab studies evaluating other indications (346–348).
In the risankizumab FORTIFY maintenance study, 297 subjects who achieved clinical response, defined as a reduction in CDAI of at least 100 points from baseline after 12 weeks of induction treatment with intravenous risankizumab in studies ADVANCE and MOTIVATE, received a maintenance regimen of risankizumab either 180 mg or 360 mg or placebo subcutaneously at Week 12 and every 8 weeks thereafter for up to an additional 52 weeks. As a consequence of the maintenance data, the recommended maintenance dosage of risankizumab is 360 mg administered by subcutaneous injection at Week 12 and every 8 weeks thereafter. It is advocated to use the lowest effective dose to treat the patient (349).
The SEQUENCE study, a prospective randomized comparative effectiveness trial, enrolled 527 patients with CD who had failed an anti-TNF agent, who were randomized to receive either risankizumab (600 mg intravenous induction at week 0, 4, and 8, then 360 mg subcutaneous injection at week 12 and every 8 weeks thereafter) or ustekinumab (intravenous dose at week 0 then 90 mg subcutaneous every 8 weeks thereafter) for 48 weeks. The coprimary endpoints were clinical remission (defined as CDAI score 1 in any individual variable) at week 48. In this study, risankizumab was noninferior to ustekinumab for the primary endpoint of clinical remission at week 24 (noninferiority margin of 10%); remission rates were reported to be 59% in the risankizumab arm and 40% in the ustekinumab arm. Risankizumab was found to be superior to ustekinumab with respect to endoscopic remission at week 48—32% vs 16% for ustekinumab-treated patients. There were no new safety signals observed in the trial (350). As a consequence of the SEQUENCE trial results, we advocate use of risankizumab as opposed to ustekinumab in patients with moderate-to-severe CD and prior exposure to anti-TNF therapy. In the SEQUENCE study, the incidence of adverse events appeared to be similar in the risankizumab and the ustekinumab group. However, the percentage of patients with serious adverse events was lower with risankizumab compared with ustekinumab (10.3% vs 17.4%); but this difference was driven largely by worsening of underlying CD. The percentage of patients with serious infections was similar in the 2 groups (3.2% in risankizumab and 4.1% in the ustekinumab group). Other studies have demonstrated similar safety findings when assessing risankizumab in patients with CD and psoriasis (351).
Recommendation
Mirikizumab is a humanized monoclonal antibody that inhibits IL-23p19 and has previously been shown to be effective in the treatment of moderate-to-severe UC (352). Subsequently, mirikizumab was evaluated in patients with CD in a phase 2 study and demonstrated efficacy to achieve clinical remission and to maintain clinical remission as well as endoscopic response. These endpoints were achieved in patients both in patients with and without previous failure to biological therapies (353). Subsequent to this study, the VIVID-1 study was initiated, a Phase 3 trial, with a treat straight through design. This global study was randomized, double-blind, double-dummy, placebo-controlled and active controlled in patients with moderately to severely active CD in patients who had intolerance to conventional or biologic therapies or who had loss of response to therapy, inadequate response, or intolerance to therapy. Patients were randomly assigned in a 6:3:2 ratio to receive mirikizumab 900 mg subcutaneously at weeks 0, 4, and 8 and then 300 mg subcutaneously every 4 weeks from week 12 to week 52; ustekinumab approximately 6 mg/kg at week 0 and then 90 mg every 8 weeks from week 12 to week 52; or placebo. In assessing the superiority of mirikizumab over placebo there were several coprimary endpoints: At week 12 PRO (patient-reported outcome), clinical response was assessed, and at week 52, CDAI (i.e., remission) and endoscopic response composite were assessed (354).
Overall, there were 1,065 patients included in the efficacy population of which 579 received mirikizumab, 287 received ustekinumab, and 199 received placebo. Mirikizumab use was effective as was demonstrated by the achieved endpoints: CDAI clinical remission was achieved in 263 (45.4%) of the 579 patients on mirikizumab compared with 39 (19.6%) on placebo (99.5% CI 15.9–35.6; P P 354).
In this study, mirikizumab was successful in achieving all individual and composite major secondary endpoints at Week 52 compared with placebo (P P 354).
There were other major secondary endpoints assessed (multiplicity controlled) that demonstrated superiority of mirikizumab over placebo including at week 12 or at week 52 CDAI clinical remission, PRO (stool frequency and abdominal pain score) clinical remission, PRO clinical response, endoscopic response, endoscopic remission, and fatigue improvement (354). These data support the use of mirikizumab for induction and maintenance of remission in patients with moderate to severely active CD.
Guselkumab is a humanized monoclonal antibody that inhibits IL-23p19 that neutralizes interleukin-23 and can bind to CD64, a receptor on cells that produce interleukin-23 which has previously been shown to be effective in the treatment of moderate-to-severe ulcerative colitis (355). Subsequently, guselkumab was evaluated in patients with CD in a phase 2 study, GALAXI-1 and demonstrated efficacy to achieve clinical remission and as well as endoscopic response (356) as well as long-term remission (357). The GALAXI-1 study was a phase 2, double-blind, placebo-controlled study, which randomized patients 1:1:1:1:1 to receive either intravenous guselkumab 200 mg, 600 mg, or 1200 mg at weeks 0, 4, and 8; intravenous ustekinumab approximately 6 mg/kg at week 0 and 90 mg subcutaneously at week 8; or placebo.
The result of the GALAXI-1 study induction phase demonstrated that at week 12 the patients in all three guselkumab treatment groups achieved a state of remission as measured by reduction in the CDAI to a level P
There was subsequently a Phase 2b study assessing the maintenance phase that began after the induction phase which began at week 12 and extended to week 48 (357). In this study patients lowered their initial guselkumab dosing from 200 mg to 100 mg every 8 weeks; from 600 mg to 200 mg every 4 weeks; from 1200 mg to 200 mg every 4 weeks. The ustekinumab group received approximately 6 mg/kg intravenously then 90 mg subcutaneous every 8 weeks; and the placebo group which had placebo induction followed by either placebo maintenance [for those with CDAI clinical response at week 12] or crossover to ustekinumab [for those without CDAI clinical response at week 12]). This study demonstrated that Crohn’s disease patients who received guselkumab via intravenous induction and subcutaneous maintenance treatment achieved high rates of clinical and endoscopic efficacy up to week 48. Additionally, the rates of clinical remission (CDAI
Another two identical phase 3 trials were performed, GALAXI-2 and GALAXI-3, evaluating the efficacy of Guselkumab in the treatment of patients with Crohn’s disease. These (358) studies were two 48-week double-blind placebo-controlled, triple dummy, treat straight through design trials which evaluated the efficacy of intravenous guselkumab given as induction therapy for the treatment of moderate to severely active Crohn’s disease followed by a subcutaneous maintenance phase. There were four treatment arms which patients were randomly entered into: 1) guselkumab 200 mg iv at weeks 0, 4 and 8 then guselkumab 100 mg subcutaneously every 8 weeks starting at week 16. 2) ustekinumab ∼6 mg/kg IV initially then at week 8 ustekinumab 90 mg sc every 8 weeks was given or placebo. 3) patients who did not have a clinical response to placebo iv at week 12 received medical therapy with ustekinumab. 4) all other patients remained on their regimens regardless of their responses at week 12. The coprimary endpoints compared placebo to each Guselkumab dosing regimen assessing the composite of week 12 clinical response and week 48 clinical remission; and the composite of week 12 clinical response and week 48 clinical remission. The primary analysis of this study evaluated 508 patients in GALAXI 2 and 513 patients in GALAXI 3. Specific endpoints evaluated in these trials include endoscopic response, endoscopic remisison, clinical remission and deep remission. Both of these trials demonstrated that guselkumab was statistically superior to ustekinumab for multiple endpoints at week 48 including endoscopic response, endoscopic remission, clinical remission and deep remission.
A subsequent phase 3 study, the GRAVITI study, was performed to assess the efficacy of subcutaneous induction and subcutaneous maintenance therapy in patients with patients with moderately to severely active Crohn’s disease (359). This study was a double-blind, placebo-controlled study that randomized patients 1:1:1 to guselkumab 400 mg subcutaneously every 4 weeks induction with subsequent conversion to 100 mg subcutaneously every 8 weeks for maintenance for a total of 48 weeks, guselkumab 400 mg subsutaneously every 4 weeks for induction with subsequent conversion to 200 mg subcutaneously every 4 weeks for maintenance for a total of 48 weeks or placebo. In this study co-primary endpoints assessed at week 12 were clinical remission and endoscopic response. There were a significantly larger number of patients at week 12 who achieved clinical remission with use of guselkumab 400 mg subcutaneously compared to placebo (56.1% vs 21.4%; Δ = 34.9; P P
Agents targeting JAK inhibitor
Recommendation
Upadacitinib, a JAK inhibitor that selectively inhibits JAK-1, is efficacious in patients with moderate to severe CD whose prior treatments have included corticosteroids, immunomodulators, or anti-TNF agents. There were 2 phase 2 induction trials (U-EXCEL and U-EXCEED) in patients with moderate to severely active CD. Approximately 45% of enrolled patients in the U-EXCEL trial had a history of prior failure of 1 or more conventional or biologic agents, and all patients in the U-EXCEED trial were required to have failed 1 or more biologic agents. Failure of therapy was defined as an inadequate response to or unacceptable adverse events as a consequence of use of medical therapy (360).
In these 2 trials, patients were randomized to receive 45 mg of upadacitinib or placebo (2:1 ratio) once daily for 12 weeks. Patients who had a clinical response to upadacitinib induction therapy were randomly assigned in the third trial, U-ENDURE maintenance trial to receive 15 mg of upadacitinib, 30 mg of upadacitinib, or placebo. U-ENDURE was a 52-week double-blind, placebo-controlled maintenance trial for patients who had a clinical response to 12 weeks of upadacitinib induction treatment in U-EXCEL or U-EXCEED. The primary end points for induction (week 12) and maintenance (week 52) were clinical remission (defined as a CDAI) score of 360).
In these phase 3 clinical trials, upadacitinib induction and maintenance therapy was superior to placebo with respect to the primary end points of clinical remission and endoscopic response as well as most secondary end points, including quality-of-life outcomes. In U-EXCEL, a significantly higher percentage of patients who received 45 mg upadacitinib compared to those patients who received placebo met the primary end points at week 12 of CDAI clinical remission (49.5% vs 29.1%, P P P P P P 360,361). The recommended maintenance dose of upadacitinib for CD is 15 mg or 30 mg daily, with 30 mg daily preferred, particularly in cases of more progressive, debilitating, or treatment-refractory (e.g., anti-TNF experienced) disease.
The safety of JAK inhibitors has been challenged with publication of the ORAL SURVEILLANCE trial, a randomized, open-label, noninferiority, postregulatory approval, safety end-point trial. This trial assessed active rheumatoid arthritis patients despite use of methotrexate who were 50 years or older with at least 1 additional cardiovascular risk factor. Patients were randomized in a 1:1:1 ratio to receive tofacitinib at a dose of 5 mg twice daily, tofacitinib 10 mg twice daily, or a TNF inhibitor. The TNF inhibitor used was adalimumab at a dose of 40 mg subcutaneous every 2 weeks (in North America, including the United States, Puerto Rico, and Canada) or etanercept at a dose of 50 mg once weekly (in the rest of the world). The use of background methotrexate was continued, unless modification was clinically indicated. The coprimary end points were adjudicated MACE and cancers, excluding nonmelanoma skin cancer. The study enrolled 1,455 patients who received tofacitinib 5 mg twice daily, 1,456 patients received tofacitinib 10 mg twice daily, and 1,451 patients received TNF inhibitor monotherapy. After a median follow-up of 4.0 years, the incidences of MACE and cancer were higher with the combined tofacitinib doses (3.4% and 4.2%, respectively) than with a TNF inhibitor monotherapy (2.5% and 2.9%). The hazard ratios were 1.33 (95% CI 0.91–1.94) for MACE and 1.48 (95% CI 1.04–2.09) for cancers. The noninferiority of tofacitinib was not shown. In the study, it was demonstrated that the risk of serious events, such as the incidence of MACE (defined as either a death from cardiovascular causes, a nonfatal myocardial infarction, or a nonfatal stroke) was higher with the combined tofacitinib doses at 3.4% than with TNF inhibitors at 2.5%, and the statistical threshold for noninferiority was not achieved. On post hoc multivariate analyses, there were several independent risk factors for MACE, irrespective of whether patients were given tofacitinib or a TNF inhibitor. These include current smoking, aspirin use, being older than 65 years, or male sex. The incidence of cancers was also higher with the combined tofacitinib doses at 4.2% than with TNF inhibitors at 2.9%, and the statistical threshold for noninferiority was again not achieved. The most common cancers were lung cancers and lymphomas with tofacitinib and breast cancers with TNF inhibitors. Cancer incidence rates were higher across all trial groups among patients aged 65 years and older. With regard to infectious complications, serious infection risk was actually only significantly elevated for tofacitinib 10 mg twice daily. Finally, when assessing tofacitinib 5 mg twice daily compared with TNF inhibitors, there was a statistically higher incidence rates and hazard ratios for deep vein thrombosis, pulmonary embolism, and venous thromboembolism. In addition, these rates go up consistently for tofacitinib 10 mg twice daily; however, this dosage is not approved for patients with rheumatoid arthritis (362).
This study served to influence US regulators to mandate prior exposure to anti-TNF therapy before allowing upadacitinib as treatment for patients with CD. There have been questions as to how generalizable these safety data are to patients with CD treated with JAK inhibitors. It is important to recognize that the ORAL surveillance trial all patients were on concurrent methotrexate, with a median dose of 17.3 mg/wk, and 57.2% of trial patients were also on background systemic corticosteroids. Nearly one-third (31.0%) of patients were older than age 65 years with mean duration of their rheumatoid arthritis >10 years, and almost half (48.2%) had a history of smoking (362). A subanalysis of ORAL Surveillance, stratifying results by age and smoking status, showed that patients younger than 65 years who had never smoked cigarettes had no increased risk of MACE, malignancy, myocardial infarction, or death among tofacitinib-treated patients relative to those treated with TNF inhibitors (363).
A recent systematic review and meta-analysis, which included 1917 patients with CD, assessed the efficacy and safety of upadacitinib and reported a pooled serious adverse event rate of 6.0%. The authors found no statistically significant differences in serious adverse event rates between the upadacitinib vs placebo group (odds ratio [OR] 0.79, 95% CI 0.62–0.99). In addition, the pooled rate of medication discontinuation as a result of having adverse events was 5.1% including opportunistic infections (0.7%) and venous thromboembolism (1.4%) (364). Upadacitinib demonstrated significant efficacy in achieving clinical remission and response in patients with moderate-to-severe CD with a low serious adverse event rate. Factoring the known risks of uncontrolled CD especially with recurrent steroid exposure, practitioners should recognize upadacitinib as an effective treatment option for the patients with CD who are resistant or intolerant to traditional immunosuppressants or TNF antagonists. Assessment of individual risk factors and careful monitoring while on treatment is essential to balance effectiveness and safety.
Severe/fulminant disease
Key concepts
Intravenous corticosteroids, dosed at methylprednisolone 40–60 mg/d or equivalent, are effective for severe to fulminant disease in the hospitalized patient (365). Pivotal trials of infliximab, adalimumab, and certolizumab pegol included patients with moderate-to-severe disease activity as indicated by the CDAI. These agents may be effective in patients with severe disease; however, it should be noted that patients with the most severe symptomatic disease, generally with CDAI scores greater than 450, were excluded (278,280,366–369). Clinical experience suggests that some patients with the most severely symptomatic inflammatory CD may respond to TNF inhibition. For more fulminant cases, infliximab may be effective, whereas the efficacy of adalimumab and certolizumab pegol in such cases is less certain. This may, in part, be attributed to the weight-based dosing used for infliximab that leads to generally higher doses than with adalimumab and certolizumab pegol and that may be more effective when there is a higher burden of inflammation.
FISTULIZING CROHN’S DISEASE
Perianal/fistulizing disease
Recommendations
Key concepts
Managing fistulizing CD presents a therapeutic challenge requiring careful evaluation and coordination of care between medical and surgical teams to ensure appropriate and timely treatment. Fistulas occur in approximately one-third of patients with CD, with perianal fistulas representing the most common location. Before initiating advanced therapy, pyogenic complications such as abscess should be excluded with cross-sectional imaging. If abscesses are present, they should be treated initially with drainage before initiation of biologic therapy or immunosuppression. Smaller abscesses may not require surgical drainage.
Perianal fistulas are categorized as either simple or complex. A simple fistula is located distal to the dentate line, primarily in the anal sphincter region with a single tract. A complex fistula can be transsphincteric, suprasphincteric, and intersphincteric in its location and may have multiple fistula tracts. This classification is important as treatments may differ among these categories. Asymptomatic simple perianal fistulas may not require medical or surgical treatment.
For symptomatic or complex fistulae, surgical consultation for exams under anesthesia are recommended as surgical drainage, fistula surgery, and/or seton placements may be necessary before initiation of advanced therapy (370). The pelvic sepsis related to fistulizing disease may lead to tissue destruction of the perianal area including the anal sphincter and more extensive perineal, gynecologic, and genitourinary complications. To that end, any fistula with an abscess or complex fistula (i.e., involving the anal sphincter, vagina, or multiple tracts) should be drained of infection. Setons are the most common method to allow for continued drainage of infection from the inflammatory fistula tracts and should be performed before initiation of immunosuppression (371). Several studies have shown the benefit of placement of setons followed by infliximab. The combination of a seton with infliximab has demonstrated a better overall fistula healing response, longer duration of fistula closure and prevention of repeated abscess, and lower overall fistula recurrence rate (372–374). In the setting of significant refractory disease, a proximal diversion to enable rectal and/or perianal healing may be necessary. After the diversion, initiation of a new therapy such as anti-TNF therapy with or without an immunomodulator may promote healing of the perineal disease. However, a systematic review suggests that the long-term success of diverting ostomy for perianal CD is very low (375). In very severe clinical scenarios, proctectomy or total proctocolectomy with permanent stoma may be necessary. Surgical advancement flaps play a role in the improvement of long-term healing rates in combination with an anti-TNF (376).
In the absence of active mucosal involvement in the rectum, patients with CD with simple fistulas may respond well to fistulotomy or mucosal advancement flap surgery, whereas patients with mucosal involvement may benefit from seton placement rather than fistulotomy with concomitant initiation of an advanced therapy: vedolizumab, anti-ILs, anti-TNF-α agents, or JAK inhibitors with the best evidence supporting the efficacy of infliximab (366,367,377–379).
Internal fistulas remain more difficult to treat. Internal fistulas may occur in the form of rectovaginal fistulas, enterovesical (or colovesicular) fistulas, or enteroenteric fistulas. Limited clinical trial data exist for internal fistulizing CD, and most of the data stem from the early infliximab studies including the ACCENT II trial which included patients with rectovaginal fistulae. Therefore, infliximab with or without an immunomodulator tends to be recommended for these patients as an initial treatment approach before surgery (377,380). The goal of medical therapy is to heal the inflamed bowel mucosa and then subsequently to enable surgical intervention. Surgical options for the treatment of rectovaginal fistulas might include excision of the fistula and the interposition of healthy tissue between the rectum and vagina. The presence of any active infection should be treated and resolved before attempting repair. After fistula excision, the treatment with a mucosal advancement flap can then be performed. For patients with enterovesicular or colovesicular fistulas, recurrent symptomatic urinary tract infection is an indication for surgery especially if associated with pyelonephritis. Surgery usually involves resection of involved inflamed bowel and closure of the bladder defect.
Enteroenteric fistulas are generally asymptomatic because they tend to form as sequelae of luminal inflammatory activity and typically do not require surgical management. Larger, symptomatic internal fistulas (e.g., stomach to ileum; mid or proximal small bowel to colon) can be associated with diarrhea, malnutrition, or small intestinal bacterial overgrowth and may require more intensive management with nutritional support and medical and surgical interventions. The presence of high-output fistulas typically mandates surgical intervention (proximal bowel diversion, bowel segment resection, or surgical fistula closure) and historically do not close spontaneously or with medical therapy.
A variety of different medications have been used to treat fistulas in patients with CD. Mesalamine and corticosteroids are ineffective treatments for fistulizing CD. Antibiotics may be used for simple, superficial perianal fistulas with minimal penetration of sphincter musculature. Typical dosing strategies include metronidazole (10–20 mg/kg/d orally for 4–8 weeks) and/or ciprofloxacin (500 mg orally twice daily for 4–8 weeks) or levofloxacin (500–750 mg once daily for 4–8 weeks) for the fistula and treatment of concurrent mucosal disease (381–384). Antibiotics also play an important adjunctive role with advanced therapies by treating the pelvic sepsis associated with more complex fistulas (385,386). However, antibiotics rarely replace the need for surgical drainage when an abscess is present.
Anti-TNF agents are effective for closure of perianal fistula, but only infliximab has been studied in a prospective, randomized controlled trial. In the initial study, infliximab 5 mg/kg at 0, 2, and 6 weeks led to complete cessation of drainage from perianal fistulae in most patients (366). A subsequent, large randomized controlled trial confirmed the efficacy of infliximab for induction of closure of perianal fistula, but also every 8-week dosing at 5 mg/kg for maintenance of complete closure and response, defined as >50% closure on clinical assessment (377). Infliximab may also be effective at maintaining response of rectovaginal fistula closure (380). Subsequent studies from clinical practice cohorts have replicated the efficacy of infliximab for the induction of perianal fistula closure and maintenance of response (387,388). Although not as thoroughly studied, adalimumab may also be effective in treating signs and symptoms of perianal fistulas. Perianal fistula closure was not a primary end point of any of the adalimumab or certolizumab studies. On post hoc analysis from 2 adalimumab CD studies, there was no benefit over placebo for fistula closure (369,389). In a large maintenance study of adalimumab for CD, fistula response and remission was a secondary end point that was achieved in a higher percentage of patients compared with placebo (278,368,390,391). A small open-label trial of adalimumab also suggested a benefit for fistula induction of remission and maintenance of closure (391). Although there are no randomized controlled trials evaluating adalimumab for induction of remission or maintenance of remission for the primary outcome of fistula remission in patients with CD, a meta-analysis of published studies suggested benefit for adalimumab use to treat fistulizing CD (392). In 2 clinical trials, combination therapy with ciprofloxacin and infliximab or ciprofloxacin and adalimumab has been shown to be more effective than monotherapy for each anti-TNF agent to treat fistulas and is effective in reduction of fistula drainage (232,386).
There is less cumulative evidence for the other mechanisms of action in perianal or fistulizing CD compared with anti-TNF therapies. The ENTERPRISE study, a small phase 4 trial investigating vedolizumab for patients with perianal CD, included 32 patients with CD with moderate-to-severe active disease and at least 1 actively draining fistula. Over 64% of vedolizumab treated patients achieved fistulae closure, and 46% had a reduction in fistula drainage by week 30 (393). In a post hoc analysis of the upadacitinib CD trials, more upadacitinib-treated patients had cessation of drainage and fistula closure during induction and maintenance study periods compared with placebo (379). Similarly, there is a suggestion of efficacy based on post hoc analysis of certolizumab pegol and ustekinumab trials, but no controlled studies indicating unequivocal benefit in fistulizing CD (280,368,392,394–398). Present evidence, based on a systematic review and network meta-analysis, has highlighted that certolizumab pegol is not as effective as infliximab for the treatment of perianal fistulas in patients with CD (392). In light of this, we suggest the use of infliximab over certolizumab pegol for the treatment of patients with CD with perianal CD.
STRICTURING CD
Key concepts
Historically, it has been believed that most strictures in patients with CD were not responsive to drug therapy and that surgery was reserved for patients with strictures and symptoms and/or signs of obstruction. However, many if not most strictures have both a fibrostenotic and an inflammatory component, so it is possible that medical therapy might result in improvement in symptoms and outcomes. In a single-center randomized trial of patients with CD and a known stricture (anastomotic or de novo) seen on magnetic resonance imaging or endoscopy, patients were randomized to either a high-dose adalimumab regimen (160 mg weekly x 4 then then 40 mg every 2 weeks with opportunity for dose escalation later based on disease activity) in combination with thiopurines or standard-dose adalimumab monotherapy (399). At the end of 12 months, significantly more patients in the high-dose combination therapy arm were more likely to have had an improvement in a 14-day obstructive symptom score (79% vs 64%) and radiographic improvement in stricture (61% vs 28%). Treatment failure (need for surgery, endoscopic balloon dilation, or change in medical therapy) occurred in 10% of those in intensive treatment vs 28% on standard adalimumab monotherapy. However, surgery rates were not significantly different (399). In a multicenter prospective cohort study of patients with CD with symptomatic strictures treated with adalimumab, 64% achieved treatment success by week 24 (i.e., off corticosteroids or other biologics and not requiring surgery or endoscopic balloon dilation), and at time of last follow-up (median, 3.8 years), 46% continued to do well with no bowel resection (400). In a post hoc analysis of 3 CD clinical trials (infliximab, ustekinumab, and azathioprine), 62.5% patients with nonpassable strictures, as determined by the SES-CD score, were able to achieve endoscopic improvement in terms of passable or resolution of strictures at 1 year, with over 50% of patients achieving clinical remission and 38% with endoscopic remission. However, overall clinical remission rates were lower compared with patients with passable or no strictures at baseline (401). For patients with stricturing disease, asking about frequency and intensity of obstructive episodes, restrictive changes in diet or food aversion, identifying radiologic evidence of fibrostenotic changes (e.g., prestenotic dilation), or multiple strictures is important for shared decision-making regarding surgical, endoscopic, or medical approaches. An international panel conducted a RAND appropriateness study and concluded that patients with CD with symptomatic strictures and evidence of inflammation could be treated with several different medical therapies, endoscopic balloon dilation, or surgery (402).
POSTOPERATIVE CROHN’S DISEASE: MAINTENANCE, PREVENTION, AND TREATMENT
Recommendation
Key concept
Several risk factors have been identified as either low risk or high risk for the likelihood of postoperative CD recurrence. The 3 factors that carry the greatest risk for postoperative recurrence are (i) active tobacco smoking after surgery, especially in women and heavy smokers; (ii) the presence of penetrating disease (i.e., fistulas, abscesses, and intestinal perforation); and (iii) history of 2 or more prior surgeries (403). Patients who have these risk factors should receive postoperative CD treatment to prevent future recurrence. Furthermore, although not formally studied, patients who progress to surgery despite treatment with an immunomodulator or biologic agent probably represent a uniquely aggressive CD phenotype and are also at a high risk of postoperative recurrence.
Factors associated with a low risk of recurrence of postoperative CD include older age (older than 50 years), a first surgery for a short segment of fibrostenotic disease (10 years), and never smoking (403–406).
A sensitive modality for early detection and monitoring of postoperative CD is an ileocolonoscopy, to be performed 6–12 months after surgery. The most widely used endoscopic scoring system, although not validated, is the Rutgeerts’ score and able to help predict future clinical and surgical risks (407). Rutgeerts’ scores of i0–i2a are associated with at least an 85% likelihood for remaining in clinical remission over a 2-year period and low risk for requiring reoperation, while scores of i2b–i4 are associated with higher risk (407,408). Rutgeerts’ i2a scoring reflects aphthae or ulcers limited to the anastomosis itself which may lower risk for disease progression, whereas Rutgeerts i2b indicates aphthous erosions or ulcers extending into the neoterminal ileum, suggesting greater severity and an elevated risk for postoperative CD progression. Rutgeerts i3 and i4 disease reflect more disease activity with diffuse ileitis, larger or deeper ulcers, and/or stricturing (174). In the Postoperative Crohn’s Endoscopic Recurrence trial, endoscopic and treatment adjustments based on a Rutgeerts’ score of i2b or greater significantly reduced the risk for subsequent clinical recurrence by 18% and 27% for endoscopic recurrence (409). A systematic review of CD studies comparing colonoscopy vs no colonoscopy–based postoperative surveillance strategies was limited by heterogeneity of studies but concluded a colonoscopy-based approach could decrease both clinical and endoscopic postoperative CD recurrence (410).
Monitoring FC plays an important role in the postoperative management of CD, serving as a non-invasive biomarker to detect inflammation and predict disease recurrence. Elevated FC levels correlate strongly with endoscopic recurrence, making it a valuable adjunct or alternative to colonoscopy, particularly for patients unable to undergo frequent endoscopic evaluations. Studies have shown that FC levels above 100–150 μg/g are associated with an increased risk of endoscopic recurrence, allowing for timely therapeutic adjustments as needed (include references here—see in comments). While colonoscopy remains the gold standard for assessment of postoperative CD recurrence, incorporating FC monitoring into routine care can reduce the need for invasive procedures and enhance early detection of postoperative recurrence (411,412).
Recommendation
Metronidazole (20 mg/kg daily) may significantly reduce the incidence of severe (Rutgeerts i3-4) endoscopic recurrent disease compared with placebo-treated patients at 3 months after surgery and clinical recurrence at 1 year (413). In a meta-analysis of clinical trials including antibiotics as post-operative prophylaxis, the use of nitroimidazoles was effective for reducing risk of clinical (RR 0.23) and endoscopic (RR 0.44) recurrence compared to placebo, however, adverse effects were common (RR 2.39) impacting treatment persistence (414). In placebo-controlled trials, nearly 50% of patients were intolerant to the imidazole antibiotics, and this postoperative prevention strategy is not sustainable for most patients. Combining metronidazole (1 g/d) for 3 months with azathioprine (100–150 mg/d) for 12 months reduces endoscopic recurrent disease (i2–4) at 1 year after surgery compared with those patients receiving metronidazole alone (415). While antibiotics ± immunomodulators are a potential treatment approach for lower-risk CD patients, the accumulating data for the effectiveness of the other advanced therapies for post-operative prophylaxis should be considered when deciding the optimal treatment strategy. Careful risk stratification and patient selection remain paramount balancing treatment efficacy, adverse effect potential, patient and disease-related risk factors, plus costs and access to treatment.
Recommendation
Evidence from multiple randomized controlled trials and open-label studies have demonstrated that anti-TNF therapy may be the most effective treatment to prevent postoperative recurrence with the potential to change the natural course of CD after surgery (409,416–426). A meta-analysis of 10 randomized controlled trials of postoperative CD concluded anti-TNF therapy, either as a monotherapy (RR 0.13) or in combination with 5-ASAs (RR 0.30) or antibiotics (RR 0.40), was effective at reducing CD recurrence compared with placebo. Neither 5-ASAs or antibiotics as monotherapy was effective over placebo (427). In a network meta-analysis of 21 controlled trials across 5-ASAs, antibiotic, and immunomodulator treatments, anti-TNF monotherapy reduced the risk of clinical relapse (RR 0.04) and endoscopic relapse (RR 0.01) compared with placebo. Anti-TNF monotherapy was the most effective medication intervention for preventing postoperative CD recurrence, with large effect size relative to all other medication strategies (clinical relapse RR 0.02–0.20; endoscopic relapse RR 0.005–0.04). The best supportive evidence for the prevention of postoperative recurrence exists for the use of infliximab (moderate level of evidence) (428).
The REPRIVIO trial, a multicenter, double-blind, randomized, placebo-controlled study, evaluated the efficacy of vedolizumab in preventing postoperative recurrence of CD. Initiating vedolizumab treatment within 4 weeks of ileocolonic resection significantly reduced the likelihood of endoscopic recurrence compared with placebo. At week 26, severe endoscopic recurrence was observed in 23.3% of patients receiving vedolizumab, compared with 62.2% in the placebo group (P = 0.0004). These findings suggest that early postoperative administration of vedolizumab may be an effective strategy for reducing disease recurrence in patients with CD after surgery (429).
Studies investigating the efficacy of the other advanced therapies for prevention of postoperative recurrence are limited and primarily retrospective or small, single-center study design (430,431). Accordingly, anti-TNF therapy is recommended as first-line prophylactic therapy for patients at high risk for postoperative recurrence or for patients who have tried and failed or are intolerant of thiopurines. Whether combination thiopurine with an anti-TNF is more effective than monotherapy anti-TNF is not known, and the postoperative trials to date have only evaluated monotherapy. Patients with CD treated with combination infliximab and azathioprine have higher response and remission rates compared with either medication alone (292,432). The authors suggest combination therapy but acknowledge that monotherapy anti-TNF is an acceptable postoperative treatment approach particularly with appropriate therapeutic drug monitoring.
Key concept
Patients at low risk for postoperative CD recurrence are nonsmokers, do not have penetrating disease, and have never had a prior surgical resection. No treatment after surgery in this population, with subsequently performing a 6-month postoperative colonoscopy to assess for the presence of CD recurrence, would be reasonable. Patients who are nonsmokers, who have penetrating disease without a prior history of surgical resection, and who have received no prior medication should receive thiopurines with or without metronidazole and subsequently undergo a colonoscopy at 6 months. If there is endoscopic evidence of disease recurrence on the colonoscopy based on Rutgeerts’ score of i2b or higher, then anti-TNF therapy should be added. Patients who have had a prior resection within a 10-year period should receive postoperative anti-TNF therapy with or without an immunomodulator and undergo a subsequent colonoscopy at 6 months postoperatively (433,434).
When to refer to surgery
Key concepts
Surgery is required in patients with CD with intractable hemorrhage, perforation, persisting or recurrent obstruction, abscess, dysplasia or cancer, or medically refractory disease (435). The most common indication for a surgical resection of the intestine in CD is because of a small bowel obstruction from a fibrostenotic stricture (436). The second most common indication for bowel resection is related to penetrating CD (e.g., an internal fistula or sinus tract resulting in an abscess or phlegmon). Although an intestinal resection is the most definitive treatment for a stricture, a stricturoplasty is an option as a bowel-preserving measure in patients at risk for short bowel syndrome. The management of CD requires a multidisciplinary approach between the gastroenterologist and surgeon (437). Surgery is not considered to be a failure of medication, and an early surgical consultation is appropriate in patients with CD with strictures or penetrating complications.
However, some subsets of patients with CD may be considered for surgery earlier in their disease history particularly if limited to a shorter segment of bowel. In a randomized controlled, open-label study (LIR!C trial) of adults with nonstricturing, nonpenetrating, shorter segment (438).
Following the LIR!C study, long-term outcomes of both interventions and the duration of treatment effect within each group were also analyzed. Retrospective long-term follow-up data were gathered for 134 (94%) of the 143 patients who participated in the LIR!C trial—69 in the resection group and 65 in the infliximab group. Outcomes of interest included the need for surgery or repeat surgery, the use of anti-TNF therapy, the duration of treatment effect, and factors influencing the duration of treatment effect. The treatment effect was defined as the time without requiring additional CD-related treatments, including corticosteroids, immunomodulators, biologics, or surgery. The duration of treatment effect was similar between the 2 groups. In the resection group, 18 (26%) of the 69 patients initiated anti-TNF therapy, with none requiring a second resection; 29 (42%) of the patients did not need additional CD-related medications. In the infliximab-treated group, 31 (48%) of the 65 patients underwent a CD-related resection, while the remaining 34 patients either maintained, switched, or escalated their anti-TNF therapy. In both groups, concurrent use of an immunomodulator along with the assigned treatment was associated with a longer duration of treatment effect (hazard ratio 0.34 in the resection group and hazard ratio 0.49 in the infliximab group) (439).
In another study using linked national registries that identified all individuals diagnosed with ileal and ileocecal CD between 2003 and 2018 who received either an ICR or anti-TNF therapy within 1 year of diagnosis, the primary outcome composite of 1 or more of the following: CD-related hospitalization, use of systemic corticosteroids, CD-related surgery, or perianal CD was evaluated. A total of 45.4% underwent an ICR and 54.6% received anti-TNF therapy. The composite outcome occurred in 273 individuals (incidence rate, 110 per 1,000 person-years) in the ICR group and in 318 individuals (incidence rate, 202 per 1,000 person-years) in the anti-TNF group. The risk of the composite outcome was 33% lower in the ICR group compared with the anti-TNF group (adjusted hazard ratio 0.67; 95% CI 0.54–0.83), and ICR was associated with a lower risk of systemic corticosteroid use and CD-related surgery, but not with other secondary outcomes. Five years after ICR, 46.3% of patients were on immunomodulators, 16.8% were on anti-TNF, 1.8% had undergone another resection, and 49.7% were on no therapy. Overall, these findings suggest that ICR could play a role as first-line treatment in the appropriate patient (440).
Recommendation
Key concept
The presence of active luminal CD with a concomitant abdominal abscess is usually the result of a sinus tract or fistula, often associated with the presence of an intestinal stricture (366). Small interloop abscesses may not be amenable to percutaneous drainage; however, most CD abscesses are accessible to ultrasonographic or computed tomography-guided drainage procedures (441–443).
The role of percutaneous drainage before abdominal surgery has remained conflicting in CD. In a meta-analysis of 9 studies including 513 patients with spontaneous intra-abdominal abscesses, the overall complication rate was higher in patients who underwent initial surgery compared with those who first underwent a percutaneous drainage of the abscess (OR 0.58; 95% CI 0.35–0.96; P = 0.03). Furthermore, the risk for recurrent abscess was higher in patients who underwent percutaneous drainage alone than those that underwent surgery (OR 2.16; 95% CI 1.03–4.54; P = 0.04) (444).
As such, once the abscess has been drained, most patients benefit from a delayed surgical resection (445). The rationale for delaying intestinal resection until the abscess is drained is because patients with peritonitis and intra-abdominal sepsis require a diverting, temporary ostomy before a surgical anastomosis is created. Some patients may benefit from a combination of abscess drainage followed by CD medical treatment, especially those with a new diagnosis and absence of stricturing disease (446,447). To date, there are no studies comparing percutaneous drainage followed by delayed intestinal resection vs medical therapy.
CONCLUSION
Significant advancements are underway in the development of new agents to address the unmet needs in treating CD. Despite existing treatments, approximately 20%–30% of patients experience primary nonresponse to anti-TNF therapies, and 30%–40% lose their response or become intolerant (secondary non-responders) within the first year of treatment. These secondary nonresponders often require dose escalation, switching to another anti-TNF agent, or transitioning to a different therapeutic class, such as anti-integrins (vedolizumab), anti-IL-12/23 agents (ustekinumab), anti-IL-23 agents (guselkumab, mirikizumab or risankizumab), JAK inhibitors (upadacitinib), or novel mechanisms (407–409).
Managing patients with refractory CD poses a considerable challenge because they often cycle through the available advanced therapies. Furthermore, a therapeutic ceiling effect has been observed in some patients, limiting the efficacy of existing treatments (410). The inability to sustain remission with current therapies has driven interest in combining biologics or small molecules with different mechanisms of action for treating medically refractory IBD. This approach aims to enhance treatment efficacy by targeting multiple disease pathways while maintaining an acceptable safety profile.
Routine monitoring of disease activity and treatment efficacy using biomarkers such as FC, CRP, and ESR is recommended. These biomarkers, however, exhibit significant variability—some patients may not have elevated CRP during active inflammation, and calprotectin levels can depend on disease location, extent, and severity. Precision medicine is emerging as a solution for CD, seeking to integrate prognostic and predictive biomarkers into clinical decision-making. Prognostic biomarkers may identify patients at diagnosis who are likely to experience more aggressive disease and require potent therapies early on, while predictive biomarkers could help match patients to the most effective treatment. The overarching goal of precision medicine is to provide the right treatment to the right patient at the right time. Several studies have been conducted in this field, including the development of clinical prediction tools for vedolizumab in CD, and combining genetic and serological markers to predict complicated CD behavior, such as the work involving tulisokibart (PRA023), an anti-TL1A agent (448–450).
Key proposals to improve CD treatment strategies include the following:
- Disease Classification: There is a need to define novel IBD subtypes and phenotypes based on molecular markers. Artificial intelligence (AI) and machine learning (ML) have been proposed to standardize and improve endoscopic, histological, and radiological assessments. A longitudinal approach, with diverse ethnic representation, is emphasized for validating biomarkers and phenotypes.
- Endpoints: Consensus on objective and reproducible clinical endpoints is critical. AI and ML can help automate and enhance these assessments, and efforts should focus on developing molecular biomarkers that correlate with disease progression and allow for frequent monitoring and timely treatment adjustments.
- Longitudinal Assessment: Ideal clinical trials would use prospective cohort designs with serial biomarker sampling. In addition, natural language processing of electronic health records could be used to assess disease phenotype, treatment exposure, and outcomes, benefiting both research and patient care.
- Clinical Translation: Companion diagnostic tests, commonly used in oncology, are essential for predicting therapeutic responses in CD. Industry-backed research programs are needed to ensure the development of validated, reproducible, and globally accessible biomarkers, which will facilitate biomarker-driven trials.
- Therapies: The success of precision medicine depends on effective therapeutic agents. While numerous biologics and small molecules are under development, it is crucial to optimize the use of existing agents. AI and ML can assist in optimizing drug choices and dosing, while combination therapies (biologics or biologics combined with small molecules) may be necessary for some patients. Personalized approaches, including therapeutic drug monitoring, will help improve treatment outcomes by enhancing our understanding of pharmacokinetics and pharmacodynamics.
These advancements hold great promise for improving the care of patients with IBD.
CONFLICTS OF INTEREST
Guarantor of the article: Gary R. Lichtenstein, MD, FACG
Specific author contributions: All authors contributed to the planning and/or conducting the study, collecting and/or interpreting data, and drafting of the manuscript. E.L.B. and M.D.L. were the ACG GRADE Methodologists.
Financial support: This guideline was funded by the American College of Gastroenterology.
Potential competing interests: G.R.L.: consulting, advisory board and/or speaking honoraria (CME)—AbbVie, Allergan, American Gastroenterological Association, American Regent, Annenberg Center for Health Sciences at Eisenhower, Bristol Meyers Squibb, Celgene, Celltrion, Eli Lilly, Endo Pharmaceuticals, Ferring, Focus Medical Communications, Gilead, Ironwood, Johnson and Johnson, Kabi Fresenius, Med Ed Consultants, Pharmacosmos, Pfizer, Professional Educational Resources, Prometheus, Sandoz, Vindico; research—Bristol Meyers Squibb, Johnson and Johnson; support of University of Pennsylvania IBD fellowship—Janssen Orthobiotech, Pfizer, AbbVie; editorship—Gastroenterology and Hepatology (Gastro-Hep Communications), Professional Communications, SLACK, Walters Kluwer, Springer Science and Business Media. C.Y.H.: consulting and/or advisory boards—AbbVie, Bausch Health, Bristol Myers Squibb, Genentech, Johnson & Johnson, Pfizer, Roivant, Sanofi, Takeda; educational and research grants—AbbVie, Helmsley Charitable Trust, Johnson & Johnson, Pfizer, Takeda. A.A.: consulting, advisory board and/or speaking honoraria—AbbVie, Bristol Myers Squibb, Eli Lilly, Johnson & Johnson, Pfizer, Takeda; educational and research grants—AbbVie, Bristol Myers Squibb, Eli Lilly, Johnson & Johnson, Pfizer, Takeda; board member and co-founder of IBD Horizons and Scrubs & Heels Foundation. M.D.L.: consulting—AbbVie, Janssen, Lilly, Bristol Myers Squibb, Pfizer, Takeda, Prometheus, Celltrion, Intecept, Roivant, Target RWE; research support—Pfizer, Takeda, Lilly. E.L.B.: consultant and/or advisory boards—AbbVie, Boomerang, Eli Lilly, Takeda, TARGET RWE. K.L.I.: consulting—Bioquest; data safety and monitoring board—Vedanta. E.V.L.: consulting and/or advisory boards—AbbVie, Abivax, Amgen, Astellas, Avalo, Biocon, Boehringer Ingelheim, Bristol-Myers Squibb, Celltrion, Eli Lilly, Fresenius Kabi, Genentech, Gilead, Iota Biosciences, Iterative Health, Janssen, Morphic, Ono Pharma, Protagonist, Surrozen, Takeda, TR1X Bio; research support—AbbVie, Genentech, Gilead, Janssen, Takeda; shareowner—Exact Sciences, Moderna.
REFERENCES







