INTRODUCTION
The liver is the largest organ in the body, and it is possibly the most complex organ in metabolism. It is comprised of multiple unique cell types having differing metabolic functions, and it has a dual blood supply, including the portal vein, carrying nutrients and gut-derived metabolites. Thus, it should not be surprising that the liver plays a pivotal role in nutritional health. There is a strong positive association between the severity of liver disease and malnutrition. Unfortunately, malnutrition is not always recognized in patients with liver disease. This is due to multiple factors. For example, weight loss in these patients can be masked by fluid retention, or patients can be obese but still have muscle wasting (sarcopenic obesity). The loss of glycogen stores predisposes patients with advanced liver disease to enter a starvation state within a few hours of fasting and that can lead to further protein catabolism. Therefore, it is important to recognize malnutrition and initiate nutrition support early in these patients. There have been over a dozen guidelines and guidance recommendations on malnutrition and liver disease by various societies over the past 2 decades. The majority addressed advanced liver disease or its complications such as hepatic encephalopathy (HE). These American College of Gastroenterology (ACG) Guidelines attempt to provide concise nutritional recommendations for the spectrum of the most prevalent liver diseases, including the newly termed spectrum of steatotic liver diseases. This new terminology will be used throughout this text (1). These ACG Guidelines will review definitions, diagnosis, causes, prevalence, prognosis, and nutritional interventions in patients with malnutrition and liver disease.
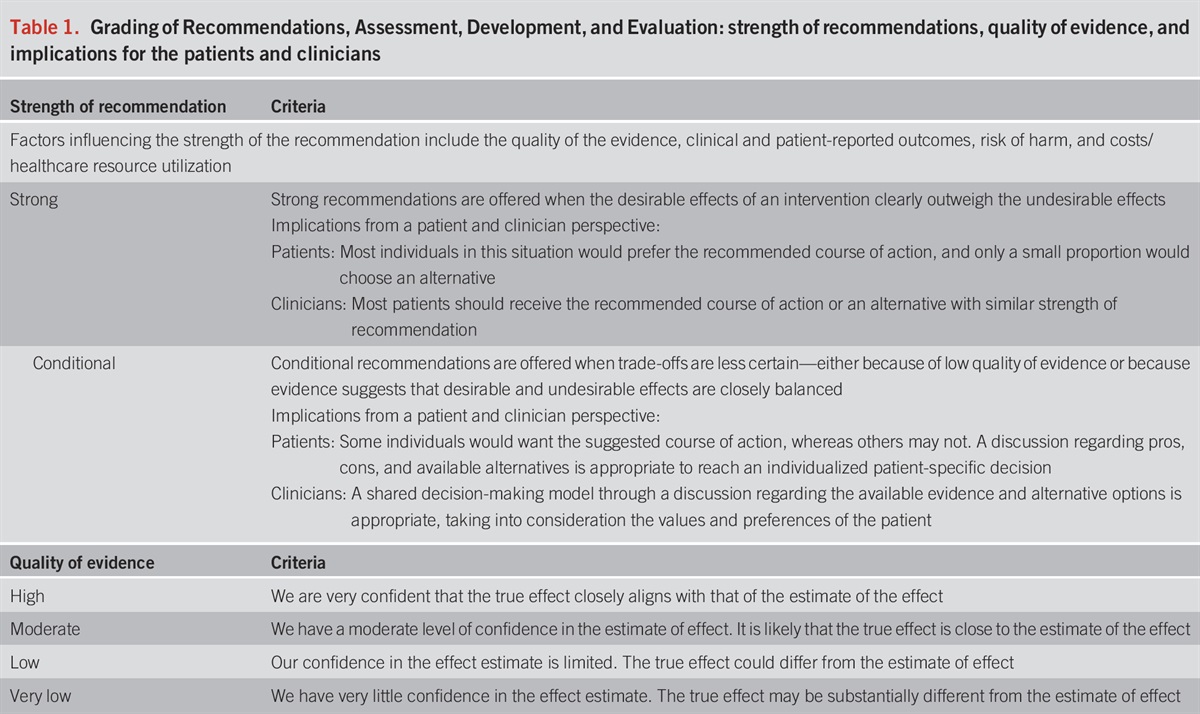
These guidelines are presented in the format of statements that were deemed to be clinically important by the content authors. The Grading of Recommendations, Assessment, Development, and Evaluation (GRADE) process was used to assess the quality of evidence for each statement (1–4) (Table 1). The quality of evidence is expressed as high (we are confident in the effect estimate to support a particular recommendation), moderate, low, or very low (we have very little confidence in the effect estimate to support a particular recommendation) based on the risk of bias of the studies, evidence of publication bias, heterogeneity among studies, directness of the evidence, and precision of the estimate of effect (2). A strength of recommendation is given as either strong (recommendations) or conditional (suggestions) based on the quality of evidence, risks vs benefits, feasibility, and costs considering perceived patient-based and population-based factors (5). Furthermore, a narrative evidence summary for each section provides important definitions and further details for the data supporting the statements.

Grading of Recommendations, Assessment, Development, and Evaluation: strength of recommendations, quality of evidence, and implications for the patients and clinicians
Under the auspices of the ACG Practice Parameters Committee, a group of experts in nutrition in liver diseases were identified for the writing group. The proposed writing group was reviewed by the ACG Practice Parameters Committee and the ACG leadership, and the final approved writing group consisted of the current authorship team, which includes hepatology experts across a broad range of practice settings and different stages of clinical and research career development.
Regular meetings were conducted among this writing group throughout the guideline development process to formulate PICO questions that guided the subsequent literature search, development of recommendation statements and key concepts, GRADE assessments, and the preparation of the full guideline document.
Electronic literature searches were conducted in PubMed (MEDLINE), EMBASE, and the Cochrane Library beginning in April 2021. The search strategy was developed and executed in PubMed (MEDLINE) and then adapted to the syntax and controlled vocabulary of Embase and Cochrane Library. The searches were filtered to fully published articles on human populations in the English language, with a focus on the highest levels of evidence. Priority was given to systematic reviews and meta-analyses, followed by randomized controlled trials (RCTs) whenever available. For PICO questions necessitating inclusion of observational studies and articles predating 2002, supplementary database searches, cited reference exploration, and manual selection by content experts were used. To ensure comprehensive coverage of literature published during the screening and review process, search updates were performed until June 2023 following the same selection criteria.
In addition to guideline recommendations, the authors have highlighted key concept statements that are not included in the GRADE assessment. Key concepts are statements to which the GRADE process has not been applied and can include both expert opinion recommendations and definitions/epidemiological statements. Table 2 gives a summary of recommendations, whereas Table 3 summarizes the key concept statements.

Recommendations

Key concepts
MALNUTRITION DEFINITIONS
Malnutrition is a broad term that includes a deficiency, excess, or imbalance of nutrients that result in adverse consequences on clinical outcomes (6). Most frequently, the term “malnutrition” is used in the context of deficiency of calories and/or proteins but is also applied to overnourished individuals (Figure 1). Malnourished patients may present with global malnutrition with frailty and/or sarcopenia. They may also present with individual nutrient deficiency (e.g., zinc) and a functional manifestation (e.g., poor wound healing or skin lesions). This term, “malnutrition,” is frequently used in relation to International Classification of Diseases codes that are developed in clinical practice. Importantly, calorie deficiency can also result in nitrogen loss due to adaptive mechanisms that result in amino acid utilization from proteins for gluconeogenesis. The consequent major body composition changes that occur because of malnutrition are loss of skeletal muscle mass, or sarcopenia, and fat loss. The most frequent phenotype of the “undernourished” patient is sarcopenia, and this term relates to the clinical consequences of muscle loss, and it is a more specific term and critical component of malnutrition. The combination of reduction in muscle and fat mass is associated with an inflammatory condition or cachexia (7). The distinction between malnutrition and cachexia is challenging because the clinical manifestations and outcomes depend on both the effects of the nutrient(s) deficiency and the underlying disease that results in malnutrition/cachexia. With the obesity epidemic, the phenotype of undernourished or cachectic patient is being increasingly replaced by a sarcopenic obese subject with muscle loss but excess fat, either in absolute terms or the muscle-to-fat ratio. In these subjects, the adverse outcomes are related to both excess fat and lower muscle mass. Finally, although micronutrient deficiencies/excess also meet the definition of malnutrition, in many situations, these deficiencies are specified individually rather than being included in the broad terminology of malnutrition. We have also included critical micronutrient deficiencies in this review because of their relevance in nutritional supplementation.
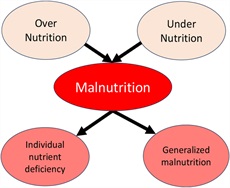
Pathways and presentations of malnutrition.
Is sarcopenia an essential component of malnutrition?
As noted above, the term malnutrition is very broad and the impact on clinical outcomes including survival, quality of life, and outcomes after hospitalization or interventions is related largely to sarcopenia. Skeletal muscle is the largest protein reservoir in humans, and an imbalance between protein synthesis and proteolysis (dysregulated protein homeostasis) results in sarcopenia (8). Calorie deficiency impairs protein synthesis. Calorie excess with an increase in fat mass initiates inflammatory and endocrine responses, which then result in both proteolysis and anabolic resistance with consequent sarcopenia (9). The impact of micronutrient deficiencies with resultant cellular/organ dysfunction also initiates adaptive and potentially maladaptive responses with consequent sarcopenia (10). Hence, sarcopenia is a critical component of malnutrition, and it is common for “malnourished” subjects to have varying degrees of sarcopenia.
Are malnutrition and/or sarcopenia dynamic processes?
Both malnutrition and sarcopenia are dynamic processes with the potential to improve or worsen.
However, clinical diagnoses of malnutrition and sarcopenia have traditionally been made at one evaluation and with no guidance on how often assessments are to be made. Importantly, in prospective studies, the effect of interventions has been observed to cause a change in lean body mass by 6–12 months (11). Retrospective analyses of skeletal muscle mass assessed by computed tomograms in patients with cirrhosis show clinically significant changes between 3 and 6 months (12).
DIAGNOSIS/ASSESSMENT OF MALNUTRITION
The assessment of malnutrition in patients with liver disease is often difficult. Some of the most used tests are given in Table 4. However, many of these tests can be influenced by the underlying liver disease. For example, anthropometry is frequently used but can be influenced by factors, such as edema and ascites. Visceral proteins such as albumin are synthesized in the liver. Subjective global assessment (SGA) is truly subjective and requires training. Multiple imaging modalities are increasingly used to assess muscle mass and body composition in cirrhosis (13–15). A single cross-sectional computed tomography (CT) slice (most often at the third lumbar vertebra [L3]) is believed to be a reliable method of assessing whole-body skeletal muscle and fat mass (15). Because patients with cirrhosis frequently have CT scans for clinical care including hepatocellular carcinoma (HCC) surveillance, CT scans may be available for body composition analysis. However, data must be extracted, which requires time and expertise. Bioelectrical impedance technology has improved, but availability is still limited. A study of 41 subjects by Pirlich et al (16) showed a strong correlation between bioelectrical impedance and the gold standard of body composition (total body potassium) for assessing malnutrition in patients with cirrhosis, including those with ascites. Untargeted approaches including proteomics and metabolomics will likely allow for easily accessible and relatively inexpensive biomarkers for sarcopenia in the future (17–19).

Clinically available methods for assessing malnutrition
Key concept
Sarcopenia is loss of muscle mass; primary sarcopenia of aging includes loss of contractile strength; secondary sarcopenia occurs in chronic diseases including cirrhosis; and compound sarcopenia refers to muscle loss in older subjects with chronic diseases (20,21). Muscle mass generally correlates with contractile strength and contractile function as measured by handgrip strength and is a good predictor of clinical outcomes including survival, risk of hospitalization, quality of life, length of stay in hospital, and discharge disposition (22). Contractile function is a better measure of muscle function and cardiorespiratory fitness than muscle mass. Hence, including handgrip strength may help predict clinical outcomes but is not necessarily a strict measure of nutritional status or muscle mass. Other measures of strength include 6-minute walk test, daily step count, and sit to stand test that have been shown to predict outcomes and depend on muscle contractile strength (23,24). Since muscle contraction and mass are interlinked (mass is needed for strength and contraction stimulates an increase in muscle mass) (25), dissecting the contribution of one without the other may limit clinical applications. Recovery of muscle strength occurs more rapidly than muscle mass, and this suggests contractile strength depends on factors beyond mass including modifications of contractile proteins and mitochondrial function (26).
Key concept
BMI (weight divided by height squared, e.g., kg/m2) is commonly used to assess health risks related to either inadequate or excessive body weight. Its advantage is that it can be automatically calculated from routinely available data in both clinical care and research. However, BMI can be confounded by increased lean body mass, which is metabolically beneficial, and current data suggest that this simple measure should be reconsidered (27). Alternatives are available, each with its advantages and disadvantages, but none as easily applied as BMI (28) (Table 4). Waist circumference correlates better with metabolic disease than BMI and should be considered in patients at risk for metabolic disease. However, it can be challenging to measure reproducibly, and in patients with ascites, waist circumference is skewed by fluid accumulation and cannot be used to assess nutritional status. An important anthropometric component of metabolic health is muscle mass and function, which can be assessed by additional methods including grip strength, thigh CT/magnetic resonance imaging/ultrasound in addition to the body composition measurements.
CAUSES
The causes of malnutrition in liver disease, especially advanced liver disease, are multiple and often interrelated (29). We highlight selected important causes of malnutrition in this population (Table 5). Patients with alcohol misuse and liver disease frequently consume approximately 50% of their calories as alcohol. These are “empty calories” devoid of critical nutrients. As summarized in Table 6, in patients with alcohol misuse, calories from alcohol remain relatively stable, while important protein intake decreases as the liver disease progresses. Recent studies show that other components of the diet are often inadequate, an example being low soluble and insoluble fiber intake in cirrhosis (30). Fiber is important for bacterial production of short-chain fatty acids in the intestine that play a critical role in gut health. Hormones and cytokines can have adverse and potentially beneficial effects. Tumor necrosis factor (TNF) can mediate muscle wasting and serum testosterone levels are often decreased in subjects with liver disease (31,32). Moreover, complications of liver disease, such as ascites and encephalopathy, can also impair adequate dietary intake.
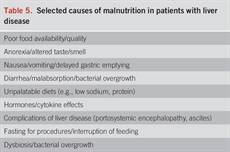
Selected causes of malnutrition in patients with liver disease

Key concepts
High-fructose intake is a potent inducer of enzymes involved in hepatic DNL, especially in individuals with the metabolic syndrome (i.e., increase in blood pressure, fasting triglycerides, fasting insulin, homeostatic measurement of insulin resistance, and decrease in high-density lipoprotein cholesterol) and insulin resistance, and are at risk for developing metabolic dysfunction-associated steatotic liver disease (MASLD). Unlike glucose, fructose metabolism is not under negative inhibition by insulin. It undergoes first pass metabolism in the liver to be converted to glucose and requires phosphorylation using hepatic adenosine triphosphate. Fructose also induces the development of hepatic steatosis through increased triacylglycerol production. Therefore, high-fructose (or sucrose) intake is associated with hypertriglyceridemia, insulin resistance, and hepatic steatosis (34). Beyond its lipogenic effect, fructose intake is also integral to mechanisms underlying hepatic inflammation and cellular stress, such as oxidative and endoplasmic reticulum stress, which are key to the progression from benign steatosis to steatohepatitis and hepatic fibrosis (35).
A randomized, controlled trial of 74 healthy adult men who were administered 200 g fructose daily for 2 weeks demonstrated onset of features of the metabolic syndrome with associated increase in liver enzymes (36). In a recent prospective study (37) evaluating increases in fasting serum fructose level (which mostly reflects endogenous fructose), the prevalence of MASLD increased for each quartile fructose level by 27.0%, 25.0%, 37.4%, and 44.5%, respectively (P P 37). Excessive fructose consumption increases the risk of MASLD, metabolic dysfunction-associated steatohepatitis (MASH), and hepatic fibrosis independent of calorie intake in a dose-dependent manner (38–40). In addition to the direct hepatic effects of fructose, indirect effects of fructose (i.e., decreased satiety, and alternations in gut barrier function/microbiota, muscle, and adipose tissue) may further lead to acquisition and progression of MASLD.
In contrast to a high-fructose intake, low-fructose diets have emerged as a strategy to mitigate MASLD. A diet low in free sugar can reduce hepatic steatosis and fibrosis while improving glycemic indices (fasting blood glucose and homeostatic measurement of insulin resistance), decreasing the concentrations of biomarkers of inflammation (high sensitivity C-reactive protein, TNF-α, and nuclear factor k B), liver enzymes, and lipids in overweight and obese patients with MASLD (41–47).
A systematic review and meta-analysis of controlled trials of the effect of fructose-containing sugars by food source at different levels of energy control on MASLD markers has been conducted (51 trials, 75 trial comparisons, n = 2,059). The evidence provides a good indication that the addition of excess energy from sugar-sweetened beverages leads to large increases in liver fat and alanine aminotransferase (ALT). However, the evidence is weaker regarding aspartate transaminase (AST) levels on the benefit of the removal of energy from mixed sources including sugar-sweetened beverages.
Finally, the association of simple sugar intake on more severe disease progression/regression was also evaluated in 80 consecutive cirrhotic liver transplant candidates who underwent detailed nutritional evaluations, and who were counseled to follow current nutritional guidelines (48). Compared with the baseline, the model for end-stage liver disease (MELD) score of cirrhotic patients at the end of the 6-month study was decreased, stable, or increased in 36%, 8%, and 56% of patients, respectively. DELTA-MELD was positively and independently correlated with the daily intake of simple sugars (48). Positive associations were present almost exclusively in patients with a visceral fat value above the median value. This is an association study, and future intervention studies are necessary to determine whether patients with cirrhosis with increased visceral adipose tissue benefit from dietary reduction in simple sugars (49).
Key concept
Anorexia is generally defined as the loss of the desire to eat, and it is usually associated with reduced food intake and deterioration of nutritional status (50). Anorexia can also be associated with poor quality of food intake. Alcohol or sugared sodas may replace necessary dietary nutrients. In patients with cirrhosis from any etiology, decreased food intake and anorexia can be multifactorial including gastroparesis resulting from portal hypertension, ascites, dysgeusia from micronutrient deficiency, and reduced taste (51,52). In studies from the Veterans Administration (VA), more than 60% of patients with moderate or severe alcohol-associated hepatitis (AH) had anorexia, and anorexia correlated with the severity of liver disease (33). Dietary protein and calories decreased as liver disease severity increased, and about half of total calories were derived from alcohol. Importantly, protein intake decreased in association with increasing anorexia (Table 6). In an outpatient study of 200 patients from Eastern India, 100% of those with alcohol-associated cirrhosis (AC) reported anorexia (53). A study from China reported that about one-third of patients with cirrhosis had anorexia, and anorexia correlated with the presence of depression. The major cause for cirrhosis in this study was viral-related (hepatitis B or C) (54). Thus, anorexia was observed in patients with different types of liver diseases across the world. Anorexia can also be caused by nutritional deficiencies. A recent study evaluated zinc status and possible symptoms of zinc deficiency in 578 patients with liver disease. Anorexia and taste disorders were associated with liver disease and were most common in patients with the lowest serum zinc levels (55). Anorexia is more common with aging, and patients with advanced liver disease tend to be older (50). Moreover, many of the presumed causes of anorexia of aging, such as inflammatory cytokines, impaired taste, and hormonal dysregulation are present in liver disease (56). Proinflammatory cytokines, such as TNF, are frequently increased in liver disease and can cause anorexia. Similarly, hormones that modulate appetite, such as ghrelin and leptin, are altered in liver disease and are believed to play a role in the anorexia of liver disease. Nausea and bloating are frequent complaints in patients with advanced liver disease and can play a role in anorexia. In a large group of patients with cirrhosis who were not eligible for transplantation, 58% had nausea (57). Gastroparesis is frequent in patients with cirrhosis; indeed, one report showed that 95% of patients with cirrhosis had gastroparesis. Gastroparesis can cause anorexia with decreased and poor-quality food intake (58,59). Patients who have complications of liver disease, such as ascites or HE, are more likely to have anorexia with inadequate food intake. Anorexia with inadequate and possibly poor-quality food intake can affect outcome. For example, in a VA Cooperative Study of 245 inpatients in which 1 month daily caloric intake was available, the calories consumed correlated in a dose-dependent fashion with mortality at 6 months (60).
Key concept
Although dysbiosis may not be directly considered as malnutrition, it is highly affected by nutrition and affects nutrition in patients with liver disease and is thus reviewed here. Because treatments for dysbiosis are frequently not nutrients (e.g., antibiotics and phages), treatment will be mentioned but not comprehensively reviewed here. The human gastrointestinal tract contains more than 100 trillion microorganisms including bacteria, fungi, viruses, and archaea that make up the gut microbiome (61). These microorganisms interact in a symbiotic fashion with the gut, liver, and immune system. Dietary factors, such as high-fat diets, alcohol consumption, and other environmental factors including chemicals, can adversely affect microbial communities. This dysbiosis can lead to intestinal barrier dysfunction, translocation of microbial components, and fecal metabolites to the liver, and the subsequent development or progression of liver disease (61). Indeed, there is substantial evidence for dysbiosis playing a role in the development/progression of very common liver diseases such as alcohol-associated liver disease (ALD) and MASLD, as well as rarer conditions such as primary biliary cholangitis and cholangiocarcinoma (61–63). Multiple experimental studies have shown that feeding mice alcohol, a high-saturated fat diet, or chemical toxins alter gut barrier function and gut bacteria, and this was associated with the development of liver injury. Importantly, studies in mice reported that alcohol-induced liver injury could be transferred into gnotobiotic mice by fecal transplant (64,65). Alcohol consumption in rodents and humans can result in gradual damage to gut barrier components leading to increased intestinal permeability and intestinal dysbiosis, including pathogenic microbial overgrowth and translocation of bacterial and fungal pathogen-associated molecular patterns (PAMPs) or endotoxins into the systemic circulation and to the liver. There are several PAMPs associated with ALD, ranging from the well-known endotoxin, lipopolysaccharide from Gram-negative bacteria, to the more recently discovered bacterial toxin, cytolysin, secreted by Enterococcus faecalis (66), and the fungal exotoxin, candidalysin, produced by Candida albicans (67). PAMPs enter the portal circulation and stimulate hepatic production of TNF and other proinflammatory cytokines through toll-like receptor-4 signaling, resulting in severe inflammation (68). This may also stimulate profibrotic changes (69), which, along with impaired liver capacity for pathogen clearance and alterations in hepatic metabolism, play a critical role in the development and progression of ALD. Endotoxemia is a common feature of patients with ALD. Studies in long-term heavy alcohol consumers with similar levels of alcohol consumption (∼15 drinks per day for ∼15 years of heavy drinking) showed that those with liver injury (based on ALT levels >40 U/L) had elevated blood lipopolysaccharide, as compared with heavy drinking individuals without liver injury (70). This suggested that low-grade endotoxemia may play a role in the development of liver disease in humans. Elevated levels of cytolysin and candidalysin, which can cause direct hepatocyte cell damage and death, were found in patients with more advanced stages of ALD, such as AH (66,67). Fecal positivity for the cytolysin-producing E. faecalis and the candidalysin-coding gene ECE1 are associated with more severe disease and mortality in patients with AH (66,67).
Like ALD, MASH was also shown to be transmissible by fecal transplant in mice (61,64,71,72). Multiple reviews have reported the association of MASLD and dysbiosis (73–75). The occurrence of small intestinal bacterial overgrowth syndrome has been reported in patients in MASLD/MASH in a cross-sectional study (76). The prevalence of small intestinal bacterial overgrowth syndrome in patients with MASLD has also been reported in a systemic review, meta-analysis, and meta-regression (77). Differences in mechanisms for dysbiosis and gut barrier dysfunction have been compared between alcohol-induced and diet-induced liver disease (78). Fecal virome transplantation can alter fecal microbiota and drive lean/obese body phenotypes in mice (79). Moreover, any alcohol use in patients with MASLD is associated with significant changes to the intestinal virome (80). Thus, alteration in bacteria, fungi, and viruses has all been associated with various types of liver disease. Finally, gut-derived metabolites in MASLD have been reported to play a role in disease modulation (e.g., indoles and short-chain fatty acids) or disease complications (e.g., ammonia and encephalopathy, trimethyl amine, and heart disease) (61).
Diverse approaches to modify dysbiosis have been used to treat liver disease or its complications (e.g., HE) (81,82) (Table 7). Probiotics have been used with some success in pilot trials in both ALD and MASLD (83,84). Fecal transplant also has shown some exciting results in treating acute AH, cirrhosis, and HE, as well as underlying drinking behavior (85,86). On the other hand, a recent large trial of antibiotic therapy showed no benefit in AH (87). By contrast, the antibiotic, rifaximin, is well-accepted therapy for HE, as well as the prebiotic, lactulose (88,89). These clinical therapeutic intervention studies further validate the importance of dysbiosis in the development/progression of liver disease and its complications. However, approaches with different endpoints in different liver diseases/complications have yielded varying results. More research in a much more personalized approach is required. Dysbiosis therapy in liver disease/complications has promising potential as demonstrated by the efficacy of lactulose and rifaximin for HE. However, dysbiosis therapy as a specific form of therapy for liver disease/complications (other than HE) cannot be recommended at this time.

Microflora modulators
PREVALENCE
Malnutrition is common in liver disease and is affected by disease type and severity, method of assessment, and whether individual nutrient deficiencies were evaluated. In some studies of patients with severe AH, every patient had some degree of malnutrition (60).
Key concept
The caloric content from alcohol consumption is substantial in AUD with or without ALD, and the alcohol intake may contribute to approximately 50% of daily caloric intake (90,91). However, these individuals are at risk for protein calorie malnutrition and deficiencies of minerals and vitamins because the calories contributed by alcohol are empty calories and do not contribute to the nutritional status (51,90). Indeed, patients with AUD admitted to treatment programs are often drinking 12–17 standard drinks per day. Owing to increasing prevalence of obesity worldwide, the current average BMI in patients with ALD in the United States is 30, with many overweight and obese patients with ALD having evidence of malnutrition and sarcopenia (sarcopenic obesity) (92). Sarcopenia and muscle loss are an objective measurement of malnutrition, and sarcopenic obesity in these patients is associated with a higher mortality compared with patients with cirrhosis without sarcopenia (93,94). Thus, in the current “real world” setting, the malnutrition seen in these patients may be driven by both obesity and empty calories from alcohol.
The risk of ALD and AH in individuals with AUD is due to the toxic effect of alcohol on the liver directly and indirectly through the gut-liver axis and can occur in the absence of malnutrition (91,95). Early studies in healthy volunteers fed alcohol in a clinical research center showed that daily heavy drinking for 2 weeks caused fatty liver (96). In a study from India, the prevalence of malnutrition was similar in individuals with AUD comparing those with ALD with those without ALD (91). In another observational multicenter study of veterans with symptomatic AH and jaundice, malnutrition was present in all the patients with severe AH vs only 62% of age-matched healthy veterans with AUD but without AH (33,60). In a retrospective study of 261 liver transplant recipients for ALD cirrhosis, malnutrition, as assessed by SGA, was present in 84% before receiving transplant (97). In another study in patients with severe AH who present with jaundice, malnutrition was universally present, and its severity increased with AH severity and hepatic complications such as ascites, HE, variceal bleeding, and hepatorenal syndrome (33,51,60,90,98,99). Studies in patients with AUD admitted to a treatment program reported very heavy drinking, but no evidence of global malnutrition in those with normal liver enzymes. On the other hand, those patients with AUD having increased liver enzymes frequently had subtle malnutrition, such as alterations in plasma lipids, zinc, or magnesium (100). Thus, although there are some data showing malnutrition in AUD without liver disease, the data are more robust showing that the risk of malnutrition increases with the development of ALD, and the prevalence increases with the severity of ALD.
Key concept
Multiple studies from around the world indicate that there is greater/more severe malnutrition in patients with liver disease having advanced fibrosis/cirrhosis compared with those with less advanced disease, and this is irrespective of the type of liver disease. Peng et al (101) performed a comprehensive cross-sectional assessment of nutritional status in 268 patients with cirrhosis of multiple etiologies and in 386 healthy controls. Total body protein was calculated by neutron activation analysis, total body fat and bone mineral density were assessed by dual-energy X-ray absorptiometry, resting energy expenditure was defined by indirect calorimetry, and grip strength was shown by dynamometry. Dietary intakes of energy and protein were also assessed. Significant protein depletion was noted in 51% of patients and was significantly more prevalent in men (63%) than in women (28%). This sex difference occurred irrespective of disease severity or origin, and the mechanisms(s) were unclear. The prevalence of protein depletion increased significantly with disease severity as assessed by Child-Pugh grade and was associated with decreased muscle function. Of interest, in this study, poor nutritional status was not related to reduced energy and protein intake (101). Several other studies compared nutritional status in patients having either ALD or non-alcohol-induced (especially viral) liver disease (91,102–105). Research from India studied patients with AUD with or without ALD, patients with cirrhosis unrelated to alcohol, and healthy controls. Alcohol constituted about 48% of daily caloric intake in patients with ALD. The percentage mean intake of carbohydrate, protein, and energy was decreased in all study groups compared with controls. The deficiencies were more pronounced in patients with severe ALD than in those with moderate ALD. Interestingly, in this one study from India, protein energy malnutrition was common in both alcohol-related and non-alcohol-related cirrhosis but was more pronounced in the latter (91). This study emphasizes the importance of cirrhosis per se in the development of malnutrition. A study from Italy (102) reported that the prevalence, characteristics, and severity of protein energy malnutrition were comparable in alcohol-related and viral-induced cirrhosis. Malnutrition correlated with the severity of the liver disease. A study from the United States evaluated patients with stable cirrhosis followed in an ascites clinic. They were not actively drinking, were free of AH, and had bilirubin levels 106). Thus, these patients with advanced cirrhosis developed malnutrition in the absence of acute inflammation. Finally, a cohort study of 52,815 men and women used bioelectrical impedance analysis to calculate appendicular skeletal muscle mass (ASM). MASLD was assessed by ultrasonography, and its severity was assessed by a fibrosis score. In multivariable adjusted analysis, the difference in 5-year change in ASM comparing participants with and without MASLD was −39.9 g (95% CI −53.1 to −26.8). When participants were further assessed by MASLD severity, ASM loss was much faster in participants with MASLD with intermediate-fibrosis to high-fibrosis scores than in those with low-fibrosis scores (107). In summary, multiple studies in different disease etiologies suggest that the severity of liver disease is critical in the development of malnutrition and muscle loss in patients with liver disease.
PROGNOSIS
Malnutrition can have a major impact on prognosis in patients with liver disease. Malnutrition can affect both complications of liver disease and overall mortality. Indeed, studies from VA Cooperative trials showed that malnutrition at admission had a dose-response effect on overall mortality at 1 month (60) (Figure 2).
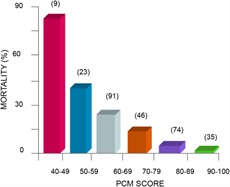
A protein/calorie malnutrition (PCM) score demonstrates that protein/calorie malnutrition relates in a graded response with mortality (a perfect score is 100). Numbers in parentheses denote the number of patients in each group.
Recommendation
Patients with cirrhosis are at risk of malnutrition irrespective of the underlying etiology (60), with a prevalence of 20% in compensated cirrhosis and up to 60% in decompensated cirrhosis (108). Five RCTs comparing EN supplementation with standard diet alone in patients with AC showed improvement in nutritional parameters and liver function (51). One of these studies reported lower patient mortality during the hospital stay with daily enteral supplementation of 2,115 calories including 71 g of protein in 16 patients compared with standard diet of daily intake of 1,320 calories in 19 patients (13% vs 47%; P = 0.02) (109). However, the other 4 studies were negative. In 3 studies, patient mortality at 1 month was 12%–13% in the intervention vs 24%–27% in the control arm (110–112). One of these studies on 51 patients (26 in the intervention arm) was among patients with cirrhosis followed in an outpatient clinic (111). The remaining study also reported no difference in outcome at 1 year (39% vs 35%, P = 0.07). This study ensured 1,800 daily caloric intake including 60 g of protein in the control arm (N = 55), and the intervention arm received supplementation (N = 44) of 30–35 calories per day (113). In a recent meta-analysis of 13 studies on 663 patients with alcohol-associated cirrhosis or hepatitis (329 intervention arm), patient mortality was lower with nutritional supplementation (21.9% vs 29%), with 20% reduced OR, 0.80 (0.64–0.99) (114). The results were similar on the route of nutrition supplementation (enteral or parenteral, P = 0.44) or study population (alcohol-associated cirrhosis or hepatitis, P = 0.07). Interestingly, the meta-regression analysis did not show any association with patient’s age, sex, daily amounts of calories or protein, and duration of intervention or follow-up. Furthermore, nutritional supplementation was associated with improved nutritional status, liver function, and decreased occurrence of cirrhosis complications especially HE.
Key concept
Malnutrition as assessed by SGA was present in 53% of hospitalized patients with cirrhosis undergoing liver transplantation (LT) and was associated with total length of stay, days spent in the intensive care unit, and total hospital cost (115). In a national cohort using the National Inpatient Sample database, protein calorie malnutrition was reported in 6.9% of all hospitalizations with cirrhosis. Compared with those without malnutrition, subjects with malnutrition had higher cirrhosis complications, in-hospital mortality (14.1% vs 7.5%), length of stay, and total charges incurred per hospitalization (116). In another observational study on 387 hospitalized patients with cirrhosis, malnutrition defined by global leadership criteria was present in 29% of patients and was associated with a 2.2- and 1.8-fold increased risk of in-hospital mortality and length of stay, respectively, compared with patients who were not malnourished (117). In a prospective study on 300 hospitalized patients with cirrhosis, muscle depletion was associated with HE at or within previous 12 months from hospitalization or with a diagnosis of minimal HE (118). In another retrospective study on 248 LT recipients, sarcopenia was present in 45% in the pretransplant period and was associated with longer length of stay (40 vs 25 days, P = 0.005) and more infections (26% vs 15%, P = 0.04), but there was no difference on median survival (9.75 vs 12.2 years, P = 0.4) compared with recipients without pretransplant sarcopenia (119). In a study on 585 candidates awaiting LT (median MELD 14), the sarcopenia present in 43% candidates was associated with higher waitlist mortality but did not improve the MELD score in predicting waitlist mortality (120). In a systematic review of 19 studies on 3,803 patients with cirrhosis, sarcopenia observed in 22%–70% studies, was associated with waitlist mortality in 4 of 6 studies, with a pooled hazard ratio (95% CI) of 1.72 (0.99–3.00, P = 0.05) after adjustment of MELD score, and was negatively associated with posttransplant survival in 6 of 11 studies, with pooled adjusted HR (95% CI) of 1.84 (1.11–3.05, P = 0.02) after adjustment of MELD score (121). Among candidates awaiting LT, subjects with cirrhosis and with frailty are more likely to experience waitlist mortality (122) and in-hospital mortality (123).
A systematic review (124) of 47 cohort studies evaluated the association between malnutrition (diagnosed using the SGA) and clinical outcomes among hospitalized patients with cirrhosis. Malnutrition prevalence varied from 5% to 100% depending on the tool used to assess the nutritional status and the study population being examined. Pooled data from 22 studies on 4,057 candidates awaiting liver transplantation showed that malnutrition was associated with pretransplant mortality with OR (95% CI) of 2.38 (1.96–2.89), with similar results found irrespective of the tool used for assessing the nutritional status. Malnutrition was also associated with cirrhosis complications including ascites, encephalopathy, and variceal bleeding. Pooled data from 6 studies on 588 liver transplant recipients showed that the presence of pretransplant malnutrition was associated with increased risk of posttransplant mortality, with OR (95% CI) of 3.04 (1.51–6.12). Malnutrition was also associated with greater length of stay including need for intensive care, infections, and packed red blood cell requirement.
Key concept
As mentioned earlier, sarcopenia (or loss of muscle mass) is the major component of malnutrition that most reliably predicts clinical outcomes and responses to interventions targeting nutritional status (7,29,125). A number of studies (both prospective and retrospective) have shown that malnutrition, contractile dysfunction, and sarcopenia are frequent in patients with cirrhosis who undergo LT and adversely affect outcomes before, during, and after surgery (7,25). With MASLD-related cirrhosis being one of the most frequent indications for LT, sarcopenic obesity or sarcopenia in obese patients, is being increasingly recognized clinically and contributes to poor outcomes similar to sarcopenia, but is more challenging to diagnose clinically due to the masking of muscle loss by the adiposity (126,127). Sarcopenia, including sarcopenic obesity, results in lower survival, quality of life, and worse outcomes after decompensation events (7,125). Malnourished and sarcopenic patients also have worse perioperative outcomes including longer postoperative stay, more blood product requirements, and higher risk of postoperative infections (7,128,129). Recurrence of HCC after LT is higher in patients with malnutrition before transplantation (130). Posttransplant outcomes including survival are worse in patients with sarcopenia and malnutrition before transplantation (131–133). The effect of pretransplant sarcopenia on graft outcomes and long-term infections posttransplantation is currently not known.
Exercise has been suggested to improve clinical outcomes before transplantation but the optimum type, duration, intensity, and safety of exercise training before and after liver transplantation, and the effects on outcomes are currently under evaluation (134). Endurance exercise increases cardiopulmonary fitness, while resistance training increases muscle mass and strength in cirrhosis, but adherence remains a concern.
Key concept
Recommendation
Interest in the concept of nutrition therapy for ALD was stimulated in 1948 by Patek et al (135), who demonstrated that a “nutritious diet” improved the 5-year outcome of patients with AC compared with historical controls with AC. Well-documented assessments of nutritional intake and nutritional status from VA Cooperative Studies demonstrate inadequate caloric and protein intake (57 g/d in the placebo arm at 1 month) in patients with severe AH. One-month daily caloric intake in 245 patients from VA Cooperative Study 275 correlated in a stepwise fashion with 6-moth survival (60). Nutritional supplementation through a feeding tube was then shown to improve liver function significantly in inpatients with ALD, as assessed by serum bilirubin concentration and antipyrine clearance, compared with inpatients who ate a standard hospital diet (136). A multicenter study from Spain (137) randomized patients with severe AH to receive either prednisone, 40 mg daily, or a liver-specific feeding formula containing 2,000 kcal/d through a feeding tube. This supplement was enriched in branched chain amino acids (BCAAs), was energy dense (1.3 kcal/mL), and was low in fat and sodium. The 1-month mortality was similar in both groups, but the 1-year mortality was significantly lower in the EN group compared with the glucocorticoid group, due mainly to fewer infections. A more recent multicenter trial in which patients with severe AH were given either intensive EN plus methylprednisolone or conventional nutrition plus methylprednisolone showed that the 6-month mortality (primary endpoint) was numerically but not statistically lower in the EN group (44.4%) compared with the control group (52.1%). Importantly, patients from either group receiving 138). Data from the 2011 to 2017 National Inpatient Sample showed that in more than 20,000 matched nourished and malnourished patients, the presence of malnutrition negatively affected local and systemic infections and mortality (139). Metanalysis of nutrition therapy in AH and ALD cirrhosis showed trends of improved mortality and improved portosystemic encephalopathy (PSE) with nutritional supplementation (114,140).
NUTRITIONAL TREATMENTS/INTERVENTIONS
Nutritional therapy can affect both nutritional status and disease complications/outcomes. We first discuss micronutrient deficiencies followed by discussions of more global nutritional interventions.
Decreased dietary micronutrient intake or absorption and increased excretion can lead to suboptimal nutritional status and cause a variety of complications in liver disease. Some deficiencies have also been postulated to enhance liver disease development or progression. Table 8 lists selected micronutrient deficiencies seen in liver disease. We review selected vitamin and mineral deficiencies and point out the need for correct diagnosis and treatment. We provide PICO questions on vitamin D, zinc, and vitamin E.
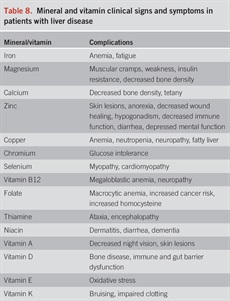
Mineral and vitamin clinical signs and symptoms in patients with liver disease
Thiamine (vitamin B1) is a water-soluble vitamin that participates in the initiation of nerve propagation and is an important cofactor for enzymes in amino acid and carbohydrate metabolism. Patients with chronic alcohol misuse or cirrhosis have been found to be thiamine deficient. Thiamine deficiency is especially prevalent in patients with alcohol-related cirrhosis due to inadequate dietary intake and through the direct effect of ethanol on thiamine uptake from the gastrointestinal tract (141). Thiamine deficiency can cause peripheral neuropathy or cardiac disease (beriberi) or neurologic impairment characterized by nystagmus, ophthalmoplegia, ataxia, and confusion (Wernicke encephalopathy). Korsakoff syndrome can also develop from thiamine deficiency and is characterized by impaired short-term memory and confabulation with otherwise grossly normal cognition. Wernicke encephalopathy is an acute disorder and requires emergent treatment, usually with intravenous thiamine.
Vitamin A plays a critical role in multiple metabolic pathways ranging from visual function to gene transcription. The liver is the major storage site for vitamin A (>90%), with most found in the stellate cells. Most studies indicate that more than 60% of patients with cirrhosis have low serum vitamin A levels and 40% in one study had impaired dark adaptation that was not recognized by the patient (142,143). A single intramedullary injection of aqueous retinyl palmitate improved night vision at retesting 1 month later, further documenting the functional nature of this nutritional deficiency. Importantly, because retinol binding protein, the carrier protein for vitamin A, is made in the liver, its serum concentration is frequently low in liver disease, which potentially increases the risk for vitamin A liver toxicity with vitamin A supplementation. Thus, supplementation in patients with liver disease/injury must be undertaken with care.
Excess hepatic copper with subsequent hepatotoxicity and fibrosis is well documented in certain liver problems such as Wilson disease and cholestatic liver diseases, including primary biliary cholangitis (144). Copper toxicity occurs through multiple mechanisms, including oxidative stress. Copper is also an essential trace element for many biological processes in the liver, including normal mitochondrial respiration, iron and lipid metabolism, detoxification of free radicals, and cross-linking of connective tissue. The American diet is marginal in copper (144). Excess fructose consumption seems to aggravate marginal copper status and induces the metabolic syndrome and MASLD in experimental animals (males are more susceptible) (145,146). Moreover, patients with MASH seem to have decreased hepatic copper (147). Thus, physicians should be aware of both copper excess and deficiency in liver disease.
Magnesium plays an important role in carbohydrate metabolism and insulin resistance (148). Magnesium deficiency can cause muscle cramps, a frequent complaint in patients with cirrhosis. Other minerals of relevance to liver disease include manganese, selenium (potential antioxidant function), and chromium (glucose tolerance). Manganese is excreted through the biliary route and can be deposited in the brain especially in the basal ganglia, with subsequent encephalopathy and extrapyramidal symptoms with features mimicking Parkinson disease (149).
Key concept
Vitamin D deficiency is common in patients with cirrhosis and has been associated with increased risk for osteopenia, osteoporosis, infections (150), mortality (151,152), and HCC (153–156). In a study of patients with chronic hepatitis B infection, the mean 25(OH)D(3) level was significantly lower than in healthy controls and was positively correlated with the albumin level. In total, 77.6% (121/156) of patients with cirrhosis had vitamin D deficiency (157). The 25(OH)D(3) level significantly decreased as the Child-Pugh classification increased in severity, was negatively correlated with the MELD score, and mortality in patients with liver failure (157). Significantly low level of vitamin D (P 158).
In women with primary biliary cholangitis (n = 118, 72% postmenopausal, 43% with cirrhosis), compared with healthy women matched for age and menopausal status (n = 472), the prevalence of osteoporosis and the yearly rate of bone mineral density loss are similar to those observed in the general population, and are not associated with the severity of liver disease (159). In a randomized trial of patients with cirrhosis (n = 164), vitamin D supplementation significantly increased 25(OH)D levels in the intervention group after 1 year (33.7 [24.3–45.7] ng/mL vs 23.1 [17–28.2] ng/mL; P 160). A recent meta-analysis showed that vitamin D supplementation alone or with calcium was not associated with reduced fracture incidence among community-dwelling adults without known vitamin D deficiency, osteoporosis, or prior fracture (161). In another meta-analysis, vitamin D supplementation with daily dose of 800 to 1,000 mg was associated with lower risks of osteoporotic fracture and fall (pooled relative risk [RR], 0.87; 95% CI, 0.78 to 0.97 and RR, 0.91; 95% CI, 0.85 to 0.98), while studies with 1,000 mg/d did not show any difference on risk of falls or osteoporotic fracture (162).
Although vitamin D3 supplementation has shown some beneficial effects in rodent models (163) of chronic liver disease, the preventive effects in cirrhosis and hepatocarcinogenesis are still unknown. In patients with ALD (n = 50), the mean level of vitamin D was 60.73 ± 28.02, 50.53 ± 39.52, and 26.71 ± 12.81 nmol/L for Child-Pugh score class A, B, and C, respectively. Supplementation of cholecalciferol 1,000 IU/d significantly increased the vitamin D levels within 6 months for Child-Pugh A and B class, with improvement in Child C after a year (P 164). A decrease in the Child-Pugh score was also found, with a redistribution of the patients in different classes. Of 7 patients initially in class C, 2 changed to class A, 4 changed from class C to B, and 1 remained in class C (P = 0.012). Of 17 patients initially in class B, 11 changed to class A and 6 remained in class B (164). Few clinical trials have shown that vitamin D supplements enhance the defense against spontaneous bacterial peritonitis with a low mortality rate in adults (158,165,166).
Recommendation
Based on a small open label pilot study in children treated with vitamin E (400–1,200 IU synthetic dl-α tocopherol daily for a mean of 5.2 months) that showed remarkable reductions in serum ALT and AST levels (167), 2 placebo-controlled, randomized trials of natural vitamin E (800 IU d-α tocopherol daily for 96 weeks) were conducted, 1 in children and 1 in adults (168,169). These trials were conducted in MASH patients without diabetes or cirrhosis and showed histological improvement in adults of 43% with vitamin E vs 19% with placebo (P = 0.001) and in children of 58% with vitamin E vs 28% with placebo (P = 0.006). Serum ALT levels improved in both adults and children, although the improvement in children was not statistically significant because the placebo-treated children also markedly improved their ALT levels. A study of patients with type 2 diabetes showed some benefits of natural vitamin E 800 IU daily alone on MASH resolution, but greater benefits when vitamin E was combined with pioglitazone (170). No improvement in fibrosis has been found in any of these studies. Meta-analyses that have included smaller studies have concluded that vitamin E treatment reduces ALT and AST levels and improves most features of liver histology except fibrosis (171–173). A reduction in serum ALT to ≤40 U/L and by ≥ 30% of baseline value with treatment has been shown to be associated with improvement in liver histology except fibrosis (174). A retrospective analysis of 236 diabetic and nondiabetic patients with MASH who were managed at a tertiary academic center was performed. Over a follow-up period of at least 2 years (median 5.6 years), 90 patients treated with vitamin E vs 90 propensity matched patients who were not prescribed vitamin E had better transplant-free survival (78% vs 49%, P P = 0.04) (175). In a study comparing diabetics with nondiabetics, the VA database was used to identify 1,572 patients with MASH who were prescribed vitamin E, and 658 had complete data for outcome evaluation over 2 years of follow-up. Mean age was 55.8 years; 283 (43.0%) patients had T2DM, and 518 (78.7%) were obese (BMI ≥30). Both diabetic and nondiabetic groups had a significant decline in mean AST and ALT over 2 years, with no significant difference between the 2 groups in their rate of change.
Clinical trials have shown that not all patients improve with vitamin E treatment and genetic polymorphisms have been identified that correlate with vitamin E responsiveness. The haptoglobin 2 allele correlates with vitamin E responsiveness, whereas patients homozygous for the haptoglobin 1 allele had no benefit from vitamin E regarding steatosis and inflammation (176). Other studies have also suggested a role of fatty acid desaturase variants in vitamin E responsiveness (177). Mechanistic data suggest that vitamin E may decrease DNL through an oxidant-sensitive pathway that regulates the activation of SREBP1c, a key transcriptional regulator of the enzymes of DNL (178).
The early clinical trial findings led to more common use of vitamin E in patients in MASH in the United States (179,180), although its use may still be relatively infrequent in Europe (181). However, enthusiasm for the routine use of vitamin E in patients with MASH has been tempered by meta-analyses suggesting that high doses may be associated with an increase in all-cause mortality (182,183); the implications of these finding for patients taking less than 1,000 U/d have not been established. In addition, increased bleeding risks with high-dose vitamin E, specifically the risk for hemorrhagic stroke, and increased prostate cancer risk have been suggested by large observational studies (184), although these findings have not yet been confirmed in prospective studies. The potential risks of long-term high-dose (e.g., 800 IU daily) use of vitamin E should be discussed with patients before initiation.
Key concept
Zinc is an essential trace element required for normal cell growth, development, and differentiation. It is involved in DNA synthesis, RNA transcription, and cell division and activation (185). It is an important component in many zinc proteins and enzymes including critical zinc transcription factors such as hepatocyte nuclear factor 4α which is vital for hepatocyte function (186). Zinc also plays a role in ammonium metabolism and ammonia removal in muscle and liver (187). Zinc deficiency is usually diagnosed by a low serum zinc level and/or clinical signs/symptoms of zinc deficiency that correct with zinc supplementation. The Japanese Society of Clinical Nutrition issued practical guidelines for diagnosing zinc deficiency, with the most important components being hypozincemia and/or clinical manifestations of zinc deficiency. Serum zinc 188) (Table 9).
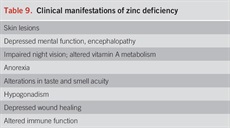
Clinical manifestations of zinc deficiency
Zinc deficiency or altered metabolism is observed in many types of liver disease, including ALD, MASLD, and viral liver disease. Some of the mechanisms for zinc deficiency or altered metabolism include decreased dietary intake, increased urinary excretion, activation of certain zinc transporters, and induction of hepatic metallothionein (185,189). Zinc deficiency is very common in patients with cirrhosis, with a prevalence as high as 84%–96% in some studies (185,190). Zinc deficiency is highly prevalent in cirrhosis patients with Child-Pugh score B or C, or with MELD score ≥15 (191). Zinc deficiency has also been shown to correlate with liver disease severity, infection, a worse transplant-free survival, and malnutrition risk (191,192). Zinc supplementation has been documented to block or attenuate experimental ALD through multiple processes, including stabilizing gut-barrier function, decreasing endotoxemia, decreasing proinflammatory cytokine production, decreasing oxidative stress, and attenuating apoptotic hepatocyte death (185,193–196).
Clinical trials in human liver disease are limited in size and quality. However, it is clear that zinc supplementation can reverse clinical signs and symptoms of zinc deficiency in patients with liver disease (185). Small trials, usually comprised mainly of patients with alcohol-related cirrhosis, have reported improved signs and symptoms such as skin lesions, taste disturbances and muscle cramps, as well as biochemical parameters including glucose disposition, insulin-like growth factor 1 concentrations, and various liver tests (197–201). Zinc deficiency may also play a role in the development of ALD. In one study, 108 very heaving drinking (≥10 drinks per day) individuals with no clinical signs of ALD or malnutrition were grouped by serum zinc concentration into normal and zinc deficient subjects. Those with hypozincemia had significantly higher liver enzymes and lower albumin, suggesting that hypozincemia may play a role in the development of early-stage ALD (202). Himoto et al (203) used polaprezinc as an antifibrotic therapy in patients with chronic hepatitis C and showed a decrease in noninvasive fibrosis markers. Subsequently, Matsuoka et al treated chronic hepatitis C virus patients for 3 years with polaprezinc, 150 mg bid. Zinc therapy was associated with improvement of AST and ALT (204).
Zinc deficiency has been associated with PSE, and zinc supplementation has been postulated to improve PSE (185,205). Severe zinc deficiency per se has been reported to cause mental disturbances and encephalopathy (185,206). In 100 patients with minimal HE, zinc deficiency predicted overt HE and mortality in patients with liver cirrhosis (205). Zinc metabolism is highly linked to ammonia metabolism, and zinc supplementation improved hepatic nitrogen clearance in patients with advanced cirrhosis (207,208). In a meta-analysis including 4 trials with 247 cirrhotic patients, zinc supplementation combined with lactulose improved number connection tests in patients with mild PSE compared with lactulose monotherapy (209). This is consistent with a previous meta-analysis of 4 trials that concluded that oral zinc improved this psychometric test but did not decrease PSE (210). If used, zinc supplementation at a dose of ≤50 mg elemental zinc orally per day should be taken with a meal to decrease the potential side effects of nausea and copper deficiency.
Recommendation
Over the past approximately 2 decades, there have been multiple publications ranging from animal data to human meta-analyses that have suggested positive health outcomes associated with regular coffee consumption. Public opinion on coffee is evolving, and it is now perceived by many to be a health-promoting beverage. Data specifically on the effects of coffee on liver health are especially compelling. It has been shown that coffee consumption is inversely related to fatty liver, advanced hepatic fibrosis, and even HCC (211–224). Studies in experimental animals have evaluated potential mechanisms, but the data are unclear (225). Individual studies and meta-analyses of data from diverse patient groups show a decrease in hepatic steatosis with coffee consumption (212–215,226). Recent data from the National Health and Nutrition Examination Survey database show that coffee consumption is associated with lower hepatic fibrosis, and the fibrosis findings are supported by other studies (216–219,221,227–229). The risks for cirrhosis and HCC also seem to be lower in coffee consumers based on large epidemiologic studies (220,223,224,230–232). It is debated whether caffeine, among more than 1,000 ingredients in coffee, is the critical ingredient for these beneficial effects (225). Some studies have reported positive effects even with decaffeinated coffee, suggesting that ingredients other than caffeine may be mediating the beneficial effects on the liver (233). Caffeine is metabolized in the liver, and reduced levels of breakdown products of caffeine correlate with the severity of liver disease (234). Thus, if caffeine metabolites were the beneficial ingredients as suggested by some studies, patients with liver disease would benefit less. Coffee consumption also is associated with epigenetic effects that may confer liver health (235). Biologically active polyphenols, such as chlorogenic acid, are present in coffee, and these polyphenols have antioxidant and anti-inflammatory properties that may play a role in the hepatic health effects of coffee (236). It seems that a wide variety of types of liver diseases derive benefit from coffee, ranging from hepatitis C to MASLD to ALD. Importantly, although coffee seems to protect against HCC, it does not protect against many other common types of cancers. There seems to be some dose-response relation, with 2 to 4 cups, often suggested as optimal amounts (233). Further studies are required to define mechanisms and dose thresholds for the hepatic benefits.
Key concepts
Fibrosis in the presence of MASH is the primary predictor of clinical outcomes (237). Lifestyle modification with diet and exercise can improve liver histology and potentially alter natural history for disease progression. Lifestyle modification is the foundation of treatment not only for MASLD/MASH but also for its associated metabolic comorbidities and improved cardiometabolic health (238). Achieving ≥10% weight loss through life-style modification may resolve steatohepatitis and improve hepatic fibrosis (239–241). Although most patients may be able to achieve modest degrees of weight loss, few patients (≤10%) achieve effective weight loss at 1 year, despite structured interventions. Importantly, fewer than half of these maintain the lost weight at 5 years after the intervention (239,242), highlighting the need for ongoing nutrition support and lifestyle modification.
Exercise independent of weight loss has a beneficial effect on MASLD/MASH. Regular moderate exercise at least 5 times a week for a total of 150 minutes per week or an increase in physical activity by more than 60 minutes per week can prevent her improve MASLD/MASH (238,243–245). More vigorous exercise is needed for resolution of MASH, with even higher intensity exercise demonstrated to reduce hepatic fibrosis (239,246).
Studies combining diet with increases in physical activity consistently demonstrate reduction in liver fat proportional to the intensity of the intervention (247–249). Even in those patients with MASH-related advanced hepatic fibrosis or cirrhosis, regular physical activity has been demonstrated to reduce portal pressure (250), improve frailty, sarcopenia, and quality of life (251). Using National Health and Nutrition Examination Survey Data (n = 3,548 participants), a dose-dependent, nonlinear association of physical activity (volume and intensity) was associated with all-cause mortality and a dose-dependent, linear association of diet quality with all-cause mortality (252). Both physical activity and diet quality were associated with lower cardiovascular risk in US adults with MASLD (252).
Key concept
Recommendation
Portal hypertension in patients with cirrhosis with consequent splanchnic pooling of blood leads to a decrease in effective circulating blood volume. Cardiac compensation with an increase in cardiac output attempts to maintain the effective circulating blood volume and renal blood flow. With increasing severity of liver disease naturally or due to a precipitant event (gastrointestinal bleeding, portal vein thrombosis, or dehydration), the cardiac compensation may not be enough, and this results in activation of renin angiotensin system with an increase in aldosterone levels, leading to active sodium and passive fluid retention. About 50% of patients with decompensated cirrhosis have fluid retention with ascites and/or edema of the feet, the most common complication of cirrhosis (253). Moderate to severe fluid retention in patients with cirrhosis is a common cause for recurrent hospitalization and utilization of resources. Hence, optimal outpatient management of patients with ascites is critical to improve patient-reported outcomes including patient survival (254).
The American Association for the Study of Liver Diseases recommends moderate dietary sodium restriction to 2,000 mg/d of salt (88 mEq of sodium) with diuretics (spironolactone with or without furosemide) as a first step in the management of clinically evident ascites in patients with cirrhosis (255). As sodium restricted diets are not very palatable and can potentially lead to malnutrition, randomized studies have been performed to examine what is the maximum sodium intake which can be allowed to these patients (254). In a multicenter study on 140 outpatients with AC, unrestricted sodium intake (n = 74) was compared with 483 mg (21 mmol) of sodium intake (n = 66) in the management of ascites. At 14 days there was a greater weight loss, decrease in abdominal girth, and improved appetite in the sodium restricted arm, but this difference was not seen at the 90-day evaluation. Furthermore, there was a trend for improved patient survival, P = 0.09 (256). In another randomized clinical trial, sodium restriction of 40 mmol/d (n = 62) was compared with 120 mmol (n = 53), with a stepped-up approach in use of diuretics in both arms. The more restricted sodium intake did not add to the 93% successful reduction in ascites with optimal diuretic treatment (257). In a more recent study from China, sodium restriction of up to 5,000 mg/d (n = 102) was compared with unrestricted sodium intake (n = 98) among hospitalized patients with cirrhosis, with use of diuretics in both the arms. The unrestricted sodium arm had higher rate of ascites disappearance and a shorter time to ascites control/discharge from hospital. Furthermore, the sodium restricted group had lower serum sodium and renal impairment during the hospitalization (258). In a larger randomized study on 328 patients managed in outpatients or during admission, 6 arms were studied with use of diuretics and other modes for management of ascites, and sodium restriction up to 500 mg/d in 5 arms and unrestricted sodium intake in the sixth arm. No statistically significant difference was seen on ascites control across the 6 arms in the study (259). Although the available evidence shows some benefit from sodium restricted diet, the contradictory studies with limited data call for larger well-designed studies to definitively examine the limit of sodium restriction in patients with cirrhosis and fluid retention.
Regarding fluid restriction, there are no high-quality randomized studies in the management of ascites and fluid retention in patients with cirrhosis. Fluid restriction is not required in the management of ascites because the fluid retention in patients with cirrhosis is passive along with sodium retention. Hence, adequate sodium control combined with optimal diuretic dose is enough in the management of ascites. However, when the serum sodium falls below 125 mEq/L due to dilutional or isotonic hyponatremia, fluid restriction is recommended by most organizations including the American Association for the Study of Liver Diseases (255).
Recommendation
Protein and caloric malnutrition is common in patients with cirrhosis, and adversely affects the clinical outcomes. Ammonia plays a central role in the occurrence of HE, a common complication in patients with decompensated cirrhosis. Ammonia synthesized by the gut bacteria from the dietary proteins is cleared by conversion to urea in the liver. However, in patients with decompensated cirrhosis, impaired liver function cannot perform this metabolic function leading to risk of HE. Hence, protein restriction to reduce the availability of substrate has been practiced minimizing ammonia synthesis (260,261). Indeed, as recently as 2001, the ACG Practice Guidelines on Hepatic Encephalopathy recommend that for cirrhotics with acute encephalopathy, protein intake should be started at 0.5 g/kg/d with a progressive increase to 1.0–1.5 g/kg/d, depending on patient tolerance (262). However, restricting proteins worsen malnutrition and sarcopenia that can potentially worsen hyperammonemia due to reduced skeletal muscle ammonia disposal (118). In a study on 30 patients with decompensated cirrhosis who were hospitalized with HE, subjects were randomized to receive protein restricted diet with progressive increment or normal protein diet for 2 weeks. The outcome of HE in the 2 groups was not different, with similar stage and severity of HE at days 7 and 14. Furthermore, patients in the low-protein intake group had higher protein breakdown compared with normal protein intake (263). In another study on 6 healthy subjects and 14 patients with biopsy proven cirrhosis (6 decompensated), plasma levels of amino acids were similar in the 3 groups after 20 g protein meal, except for greater increase in the levels of isoleucine, leucine, and tyrosine in patients with cirrhosis compared with healthy subjects. However, none of the patients with cirrhosis developed covert HE as evaluated by the number connection test (264). A large clinical trial at 8 Department of Veterans Affairs Medical Centers analyzed food intake including protein consumption and encephalopathy over time. Sixty-three percent of patients had some degree of HE at entry, which decreased over time. Time-dependent regression analysis found low-protein intake to be independently associated with worsening HE. Similarly, less malnutrition at study entry was independently associated with improved HE (265).
Recommendation
Dietary protein is a major source for ammonia production in the gut, a major player in the occurrence and/or control of HE. The recommended protein dietary intake could be derived from the vegetarian/plant-based or animal-based source. Vegetable sources of protein compared with animal sources have higher arginine content (which increases urea production), higher fiber content (which creates an acidic environment in the colon, mediating excretion of ammonia in the stools), and have lower content of methionine and tryptophan. Taken together, these effects result in lower circulating blood levels of ammonia and mercaptans, both of which are involved in mediating encephalopathy (266).
Several studies have compared vegetable vs animal sources of protein in patients with cirrhosis and HE. Of a total of 6 studies, vegetarian sources of protein were associated with improvement in 4 studies and no differences in outcomes in the other 2 studies. However, these studies are limited because they are more than 25 years old, have small samples, and used different intervention regimens (Table 10). An RCT from India randomized 120 patients with cirrhosis and minimal HE to half receiving 30–35 kcal/k/d with 1.0–1.5 g/k/d of vegetarian source of protein and 60 patients receiving no nutritional supplementation (267). After 6 months, nutritional supplementation with vegetable source of protein vs no supplementation was associated with improvement in HE (71 vs 23%, P = 0.001) as assessed by psychometric tests. Furthermore, development of overt HE was in 10% vs 22% of patients, respectively, P = 0.04.

Studies comparing the impact of vegetable vs animal source of protein on HE in patients with cirrhosis
Recommendation
BCAAs such as leucine, isoleucine, and valine are essential amino acids (diet as the only source) because these cannot be synthesized in the liver. Ammonia is a key mediator in the development of HE in patients with cirrhosis and chronic liver disease with significant hepatic insufficiency or portal hypertension with portosystemic shunting (274). Muscle plays a major role in the removal of ammonia by its conversion to glutamine through glutamine synthase (275). Patients with cirrhosis and HE have altered ratios of amino acids, with reduced levels of BCAAs and elevated levels of aromatic amino acids (276,277). BCAAs provide their carbon atom to replenish α-ketoglutarate, which is consumed to generate glutamate, an essential substrate to convert ammonia to glutamine. Furthermore, BCAA supplementation also reduces protein breakdown in patients with cirrhosis, with consequent improved muscle mass, which helps in extrahepatic detoxification of ammonia (263,274). Several RCTs have examined the benefits of BCAA supplementation on improvement of HE and patient survival. A systematic review (278) and meta-analysis by the Cochrane group of 16 RCTs on 827 patients with HE, 12 trials including overt encephalopathy and 4 minimal encephalopathy (asymptomatic patients but with abnormal psychometric tests), examined the benefit of BCAA supplementation (278). Pooled data from 9 trials on 430 patients showed improvement in HE with oral BCAAs, RR (95% CI) of 0.67 (0.52–0.88), but not when IV BCAAs were used in 7 trials on 397 patients, 0.81 (0.61–1.09, P = 0.34) (278). Pooled data from 15 trials on 760 patients showed no difference in patient mortality comparing the intervention vs control arms (21.3 vs 22.6%), RR (95% CI) of 0.88 (0.69–1.11) (278). However, after excluding 5 trials with control group of lactulose or neomycin, the risk of patient mortality was reduced by 24% with RR (95% CI) of 0.76 (0.63–0.92). Although no serious adverse effects were observed, the use of BCAAs led to side effects, especially nausea and diarrhea (11 vs 2%), 3.39 (0.7–16.46). Improved liver disease and HE probably overcame the adverse effects, with no differences on quality of life between BCAAs and control arms, as assessed by the SF-36 tool (278).
Recommendation
The importance of outpatient nutritional therapy for patients with cirrhosis was reported over 75 years ago when Patek et al (135) demonstrated that a “nutritious diet” improved the 5-year outcome of patients with cirrhosis compared with a similar group of patients consuming usual diet. Multiple studies support the concept of improved outcomes with nutritional counseling/support in patients with cirrhosis. Hirsch et al (111) showed that outpatients who supplemented their diet with an enteral product (1,000 kcal, 34 g protein) had significantly improved protein intake and fewer hospitalizations. These investigators also treated outpatients with alcohol-induced cirrhosis with an enteral supplement and observed an improvement in nutritional status and immune function (279). A study from Japan evaluated patients with cirrhosis of diverse etiology who either received nutritional counseling and intervention from a dietitian after nutritional assessment, or standard of care. Patients were followed for approximately 5 years, and those who had nutritional counseling had improved survival, especially those with Child A cirrhosis.
Late night snacks and frequent meals are an integral part of outpatient nutritional management of patients with liver disease (280). Glycogen storage is an important function of a healthy liver, which helps release glucose for energy through glycogenolysis during periods of starvation beyond 48–72 hours. Owing to reduced glycogen stores in patients with cirrhosis, shorter fasting periods of even 12 hours result in increased oxidation of fats and proteins along with activation of gluconeogenesis. Increased muscle protein breakdown and decreased muscle protein synthesis during periods of fasting results in anabolic resistance, with a negative nitrogen metabolism and sarcopenia, leading to adverse outcomes of these patients (51,90). In a study on 103 cirrhosis patients randomized to receive nutrition supplementation of 710 calories during the day between 7 am and 9 pm (n = 52) or during night between 9 pm and 7 am (n = 51), total body protein measurements were higher at 3, 6, and 12 months compared with baseline in the night time group, but no such changes were seen among patients who received the same supplementation during the day (11). Since then, several other studies on cirrhosis of varying etiology, study population, and intervention duration (short-term of 1 month or longer), timing, calories, and composition and source of proteins (regular or branched chain amino acids) have been performed to examine the benefits of late evening snacks in cirrhosis patients. Several of these studies had significant limitations, such as not being randomized, small sample size, lack of a control group, and inability to blind the groups. In a systematic review of 15 studies examining late evening snack in patients with cirrhosis, the pooled heterogeneous data showed a consistent effect in reducing muscle protein breakdown, with an improved nitrogen balance and quality of life. However, the effect on skeletal muscle mass was inconsistent across the studies, whereas no effect was reported or observed on liver transplant-free patient survival (280). In another meta-analysis of 8 studies in 341 patients, a late evening snack in 167 patients compared with 174 control group subjects was associated with improved biochemical liver profile and liver disease complications of ascites or HE (281). It should be acknowledged that the supplementary snack can affect the blood glucose level and potentially worsen the gastroesophageal reflux, if taken too close to bedtime. Frequent small meals or snacks every 3–4 hours while awake also has been reported to help maintain nutrition. Similarly, an adequate breakfast was associated with an improvement in cognitive function tests in patients with cirrhosis (282). Larger randomized studies with long-term follow-up are needed to examine the impact of nutrition counseling and late evening snack on transplant-free survival.
CONCLUSIONS
Our definition of malnutrition includes both undernutrition and overnutrition, and it includes both macronutrient and micronutrient deficiencies. Malnutrition generally increases as the severity of liver disease increases, and malnutrition is often marker of poor prognosis. Unfortunately, there are major gaps in our knowledge with only limited large studies related to malnutrition and liver disease, and there are even fewer randomized studies of nutritional interventions and liver disease. Thus, we have provided more guidance than guidelines. Some of the commonly used recommendations for nutritional support in cirrhosis are extrapolated from nutrition studies in other severely ill patients. As we learn more about nutrition and liver disease, and especially the interactions of nutrition and the microbiome and liver disease, it is likely that a “personalized approach” to nutritional therapy will evolve. Increased use of dieticians and multidisciplinary approaches are optimal. Well-designed large research trials related to nutrition and liver disease should help fill multiple existing gaps in our knowledge.
CONFLICTS OF INTEREST
Guarantor of the article: Craig J. McClain, MD, FACG.
Specific author contributions: All the authors wrote different key portions of this guideline, were involved in regular virtual meetings of the committee members, made appropriate revisions, and approved the final manuscript.
Financial support: PON27462400004956; P20GM103436; U01AA026980-06 (AKS). NIH: RO1GM119174; RO1DK113196; RO1AA021890; R01DK133905; U01DK130180; P50AA024333 (SD); DK093568-03 (MA); U01AA026934; U01AA026936; U01AA026980; P20GM113226; P20GM113226-07S2; P20GM113226-08S1; P50AA024337 (CJM); and the VA: 2I01CX002219-05 (CJM).
Potential competing interests: M.A. has received consulting fees from Corcept Therapeutics, Intercept Pharmaceuticals, and Novo Nordisk AS. B.N-T has received consulting fees from Merck Sharp & Dohme, E.R. Squibb & Sons, and GlaxoSmithKline. A.S. has received consulting fees from Siemens Medical Solutions. The remaining author reports no potential conflicts.
REFERENCES







